Meeting Summary
During this symposium, leading experts in paediatric allergy and immunology reviewed new evidence for the role of human milk oligosaccharides (HMO) in supporting the development of the infant microbiota and modulating the immune system, thereby improving the clinical management of cow’s milk protein allergy (CMPA). Liam O’Mahony, University College Cork, Ireland, explored the mechanisms by which HMOs can modify the gut microbiome and beneficially influence allergic and infectious responses in both healthy infants and those with CMPA. New data from the CINNAMON study were showcased by Claire Boulangé, Nestlé Institute of Health Sciences, Lausanne, Switzerland, highlighting key mechanisms by which specific HMOs can support the microbiome and modulate metabolome production that may lead to important immune benefits in CMPA. Finally, Anna Nowak-Węgrzyn, Professor of Pediatrics and Chief of the Division of Pediatric Allergy and Immunology at the Grossman School of Medicine, New York University (NYU) Langone Health, USA, presented results from the Platypus study, in which infants with moderate-to-severe CMPA were fed an amino acid (AA)-based formula containing two HMOs. Symptoms of CMPA decreased significantly in infants fed the HMO-supplemented formula, and these clinical improvements were accompanied by normal growth and positive changes to the faecal microbiome. Collectively, these findings translate to important immune benefits and a key role for HMO-supplemented formula in the clinical management of CMPA.
Mechanisms of Human Milk Oligosaccharides Modulating Immune Responses
Liam O’Mahony
In early life, the infant microbiome is developing alongside the nascent immune system.1 Microbiome composition and metabolic functions are critical to this early life immune development, in particular the establishment of effector and regulatory immune networks, failure of which can result in allergy.1-3
But who is controlling this orchestrated microbiome development? O’Mahony highlighted HMOs as one of the dietary factors with a key role in supporting early life microbes. HMOs are the third most abundant component of breast milk, with composition influenced by multiple factors, including genetics, gestational age, and geography. Several studies have shown that breast milk HMO composition correlates closely with allergy risk, and levels of specific HMOs associate with development of CMPA and future food sensitisations.2,3
The influence of HMOs also extends beyond allergy. Formula containing the two HMOs, 2’fucosyllactose (2’FL) and lacto-N-neotetraose (LNnt), was shown to significantly reduce the risk of bronchitis and lower respiratory tract infections in infants ≤12 months old, compared to those fed the same formula without HMOs in a randomised, controlled trial. Associated benefits of these reduced infection risks included less use of medications, namely antibiotics and antipyretics.4 Antibiotics themselves are detrimental to the early life microbiome, so reducing their use can have additional benefits.
HMOs influence the early life immune system via at least three mechanisms. Firstly, HMOs are utilised by specific protective species in the gut, notably Bifidobacteria, leading to their expansion. Experiments have shown that Bifidobacteria have potent immune-regulatory activity and can lead to induction of T regulatory cells in the gut, which has important implications for protection against allergies and infection.5-7 The positive immune benefits gained from expansion of protective Bifidobacteria early in life are mediated in part by immunomodulatory cell structures. For example, certain polysaccharides on the cell surface of Bifidobacteria have been shown to dampen allergic inflammatory responses and protect against lethal influenza infection in mouse models.8-10
The second key mechanism of HMOs is modification of microbial metabolism and generation of immune regulatory compounds. O’Mahony referenced a recent study in which HMOs fed to infants gave rise to an “enormous change in metabolism,” including increased levels of sphingolipids, important for the development of intestinal invariant natural killer T cells, reduced levels of the T regulatory inhibitor 12,13-dihydroxylated fatty acid, and changes in the gamma-glutamylation of AAs associated with reduction in inflammatory cytokines.11 The most well-described bacteria-derived metabolites with immunomodulatory activity in the gut are the short-chain fatty acids (SCFA), which comprise acetate, propionate, and butyrate. “SCFAs are really important early in life,” explained O’Mahony. “They can affect epithelial cells and modulate epithelial barrier function; influence effector cell types like neutrophils, mast cells, and innate lymphoid cells; modify cytokine secretion by dendritic cells; and exert direct effects on the expansion of regulatory cell subsets.12 We and many others have shown that increased SCFA levels correlate with decreased risk of allergic outcomes,” noted O’Mahony. One study found that approximately 56% of children with lower levels of butyrate or propionate at age 1 year had become sensitised to air or food allergens by the age of 6 years; whereas those with higher levels of SCFAs early in life had a significantly lower rate of sensitisation (approximately 20–27%).13 Other outcomes such as asthma, allergic rhinitis, food allergy, and atopic dermatitis were also lower in those children with higher levels of butyrate at age 1 year. Notably, the rate of asthma at age 6 years was halved in those children with higher levels of butyrate or propionate in early life, compared to those with the lowest levels.13 These are really important metabolites that should be supported early in life for proper immune development, stressed O’Mahony.
Finally, HMOs also exert direct effects within the gut, namely on the epithelial barrier and immune cells. One of the best-described mechanisms is the binding of HMOs to pathogens to prevent adhesion and subsequent infection by acting as decoys for histo-blood group antigens receptors. HMOs can also boost epithelial barrier function by reducing apoptosis and promoting proliferation and differentiation. They also can directly modify innate immune cell activity and can promote IL-22 secretion through effects on dendritic cells, which further reinforces epithelial barrier function in the gut.
In summary, human breast milk contains numerous immunomodulatory factors, including HMOs, which exert directly and indirectly on the immune system. Importantly, the development of both immune regulatory and immune effector systems are key in early life and are supported by HMOs, O’Mahony concluded.
2’Fucosyllactose and Lacto-N-Neotetraose Shape the Gut Microbiome and Metabolome in Cow’s Milk Protein Allergy
Claire Boulangé
There is a growing body of evidence to show that HMOs support beneficial gut microbes and the infant’s physiological development, hence a “clear interest” in including HMOs in formula specifically adapted for children with CMPA, explained Boulangé.4,14-18 Boulangé presented new data from the CINNAMON clinical trial, shedding further light on mechanisms by which HMOs positively influence the faecal microbiome and ensue metabolite production that may deliver important immune benefits in children with CMPA.16
CINNAMON was a controlled, double-blind, randomised, multicentre study, in which 194 infants were randomised to either a lactose-containing, whey-based, extensively hydrolysed formula (w-EHF) control group, or a test group fed similar formula with the addition of two HMOs: 2’FL (1 g/L) and LNnT (0.5 g/L). The primary outcome of the trial showed that HMO-supplemented w-EHF supported normal growth in infants with CMPA, and suggested a protective effect against respiratory and ear infections in the first year of life.16
A key secondary endpoint of the study was to explore the role of HMOs in the microbiome and metabolome of w-EHF-fed infants with CMPA. Faecal samples were collected at different timepoints and metagenomics, and targeted faecal metabolomics were performed to evaluate the impact of HMOs on the gut ecosystem in these infants. Infants were followed until 12 months of age. Boulangé clarified that, due to age heterogeneity at enrolment, infants were categorised into two cohorts and analysed separately: early enrolment (EE) from 0–3 months and late enrolment from 3–6 months. The latter cohort may have been exposed to solid food prior to enrolment. To characterise temporal changes in the microbiome over time, an algorithm was applied that clustered the different microbiomes into five faecal community types (FCT) based on their taxonomical composition. GC1 and GC2, enriched in Bifidobacteria, are seen earlier in life, with infants then transitioning to the later FCTs (GC3, GC4, and GC5).
Comparison of the distribution of FCTs between control and test groups showed that HMO feeding favoured early FCTs, driven by Bifidobacteria in both cohorts. This enrichment for earlier FCTs was “significantly different” in the EE cohort after just 3 months of treatment (Visit 3), highlighted Boulangé, with a significant lowering of GC3 in the HMO group versus control (p=0.025). An enrichment of early FCTs was also seen when infants were 12 months of age (Visit 6), but the difference was not significant. In the late enrolment cohort, the enrichment of earlier FCTs occurred at 12 months of age (Figure 1).
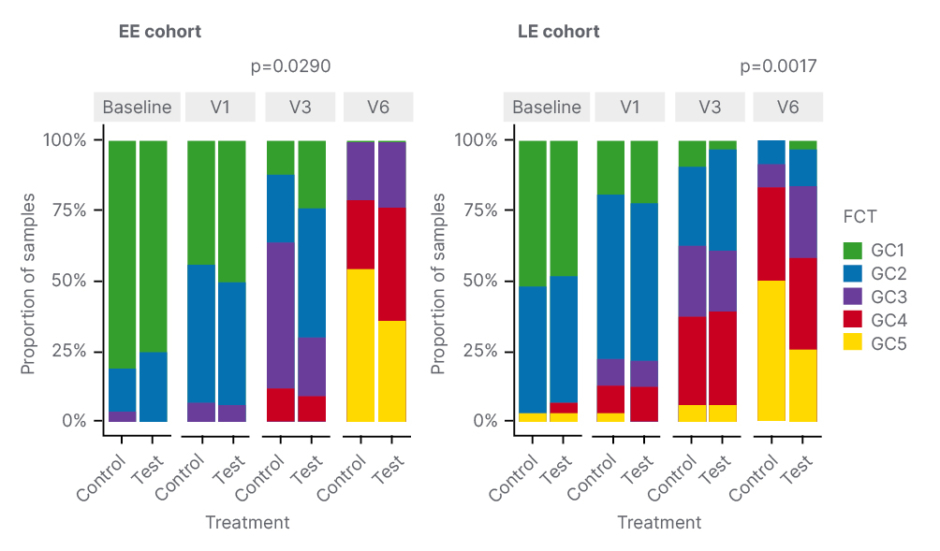
Figure 1: Human milk oligosaccharide feeding favoured early faecal community types driven by Bifidobacteria.
EE: early enrolment; FCT: faecal community type; LE: late enrolment; V: visit.
“We found that HMOs modulated the faecal metabolome in both cohorts, but the signature was significant when infants were enrolled early,” noted Boulangé, so the EE cohort was the focus for the metabolomic analysis. High levels of 2’FL and lactose were found in the test group, showing good compliance to the product. Results showed that HMO supplementation reduced faecal AA metabolites, bile acids, and phenylalanine, and may help to maintain key bacterial functions. Specifically, HMOs transiently supported faecal acetate production like those at baseline, and promoted bile acid deconjugation over time.
The next step was to combine faecal metabolome analysis with microbial functionality derived from metagenomic data. Findings demonstrated that the microbiome contributes to the production of AA derived metabolites. These AA-derived metabolites correlated positively with two bacterial enzymes from the Ehrlich pathway, which is also called the oxidative AA catabolism pathway, but were negatively associated with some enzymes originating from the biogenic amine pathway. Interestingly, both metabolites and enzymes from the Ehrlich pathway were inversely linked with HMO intake, which highlights that HMOs downregulate this oxidative AA catabolism, Boulangé explained. Also identified was a positive association between bile acid salt hydrolase, which is the bacterial enzyme responsible for the deconjugation of bile acids, and the ratio of unconjugated versus conjugated bile acid. “Overall, therefore, we can say that the faecal metabolome closely reflects HMO-related changes in the microbiome,” summarised Boulangé.
Boulangé described these findings as “particularly interesting,” because some of the associated metabolic changes have been linked to a beneficial host impact in the literature. For example, by downregulating the AA oxidative catabolism pathway, HMOs may trigger a potential switch to the carbohydrate pathway for energy production and a reduction in levels of harmful uraemic toxins involved in the proinflammatory process, such as p-cresol sulfate and phenylacetate, the former having been linked with symptoms of autism in both mice models and humans.19 HMO-driven reduction of proteolytic activity may be linked to decrease in facultative anaerobes, which are usually found in dysbiotic microbiomes.20
HMO-mediated changes in bile acids may also exert a positive impact on metabolic and inflammatory processes in the host. Bile acid salt hydrolase, which is present in multiple bacterial taxa, including bifidobacterial, is responsible for the deconjugation of primary bile acids. Increased deconjugation activity linked to HMO intake may, therefore, be associated with a boost in Bifidobacteria.21 This is important, as an increase in deconjugated bile acids elicited by HMOs may contribute to delayed maturation of the infant microbiome.22,23 Deconjugated bile acids are agonists of the farnesoid X receptor, which regulates the bile acid pool in the liver. In addition, the farnesoid X receptor modulates lipid metabolism and inflammation in enterohepatic tissues and peripheral organs.21,24
In summary, these new data from the CINNAMON study show that supplementation of a w-EHF with two HMOs in infants with CMPA slowed the faecal microbiome maturation and promoted a Bifidobacteria-enriched ecosystem. This positive effect was particularly pronounced in the cohort of infants enrolled early (before 3 months), when still on a milk-based diet, noted Boulangé. Several significant HMO-associated changes were found in the faecal metabolome of the EE cohort. HMOs appeared to down-regulate bacterial AA catabolism, which may reduce the production of potentially harmful metabolites such as p-cresol sulfate. Infants in the HMO group also maintained higher levels of faecal short chain fatty acids SCFA (namely acetate), at least transiently, which is a signature metabolite of HMO fermentation by Bifidobacteria. Finally, the 2 HMOs, 2’FL and LNnT, helped to sustain beneficial bacterial bile acid deconjugation activity over time. “We know that infants with CMPA are more prone to having a dysbiotic microbiota and to proinflammatory disorders, as well as gastrointestinal (GI) issues, and here we provide further insight that HMOs may have clinical relevance in this population,” Boulangé concluded. These metabolome effects could not be confirmed in the cohort of infants enrolled after 3 months of age, likely due to the confounding effects of dietary fibre from the weaning diet, and a progressive reduction in formula intake.
Clinical Relevance of 2’Fucosyllactose and Lacto-N-Neotetraose in the Nutritional Management of Cow’s Milk Protein Allergy
Anna Nowak-Węgrzyn
Nowak-Węgrzyn began by recapping the ‘journey’ to establish the key role of the two HMOs, 2’FL and LNnT, in the nutritional management of CMPA. First came a study confirming the safety and hypoallergenicity of w-EHF supplemented with 2’FL (1 g/L) and LNnT (0.5 g/L).25 The percentage of infants tolerating the formula was 98.4%, thereby fulfilling hypoallergenicity criteria set out by the American Academy of Pediatrics (AAP).25
Subsequent studies proved “even more interesting,” continued Nowak-Węgrzyn, and revealed a protective role of HMOs against several infections. In the CINNAMON study, infants fed a w-EHF supplemented with 2’FL and LNnT showed a clinically relevant reduction in their relative risk of several infections from enrolment to Month 12, including lower and upper respiratory tract infections, otitis media, and GI infections.16 When analysed in terms of frequency, a statistically significant 42% reduction (p=0.003) in the rate of upper respiratory tract infections was detected in the test formula group versus control.16 Overall, findings from the CINNAMON study have suggested that HMO-supplemented formula has immune-enhancing properties, conferring a protective effect against respiratory and ear infections in infants with CMPA.16 Adding the components 2’FL and LNnT that are present in human breast milk augments the anti-infectious capacity of the hypoallergenic formula, in addition to supporting normal growth, Nowak-Węgrzyn confirmed.
In additional to clinical outcomes, microbial diversity has also been studied in detail in the CINNAMON study of infants fed w-EHF supplemented with 2’FL and LNnT. Although initial findings were “surprising,” revealing lower microbial diversity at 12 months of age, additional analysis showed the variety of microbiome, which more closely resembled that of exclusively breastfed, healthy infants.26 Nowak-Węgrzyn explained that 2’FL and LNnT supplementation appeared to slow the premature shift towards an adult-type gut microbiome previously described in infants receiving no, or only some, breast milk.26-28 At 12 months, the HMO-supplemented infants also had a microbiota that was enriched in early-type FCT, more similar to that seen in breastfed infants.26
For the final part of her presentation, Nowak-Węgrzyn focused on results of the Platypus study: a single-arm observational trial conducted in Australia that evaluated growth in 32 infants with moderate-to-severe CMPA fed an AAF containing two HMOs: 2’FL (1 g/L) and LNnT (0.5 g/L).29 The mean age of the study population was 18.6 weeks, with a slight male predominance (62.5%). In terms of clinical manifestations of CMPA at enrolment, the majority of infants had GI or delayed-type skin reactions. The most common GI symptoms were irritability/crying (87.5%), regurgitation/vomiting (62.5%), and persistent diarrhoea (59.4%). Eczema/atopic dermatitis was present in 53.1% of infants.29
Results of the Platypus study showed improvement in growth parameters with HMO-supplemented formula feeding.29 All anthropometric parameters (i.e., Z-scores for weight, length, head circumference, and BMI) progressed along the World Health Organization (WHO) normal growth curves, with an upward trend from baseline to 4-month follow-up (Figure 2). Nowak-Węgrzyn also noted that there was a “dramatic improvement in symptoms,” with infants experiencing a significant reduction in the frequency of presenting CMPA symptoms from enrolment to 1-month follow-up (Figure 2). Overall, the study AAF demonstrated an excellent safety profile and was tolerated well by infants with moderate-to-severe CMPA.29
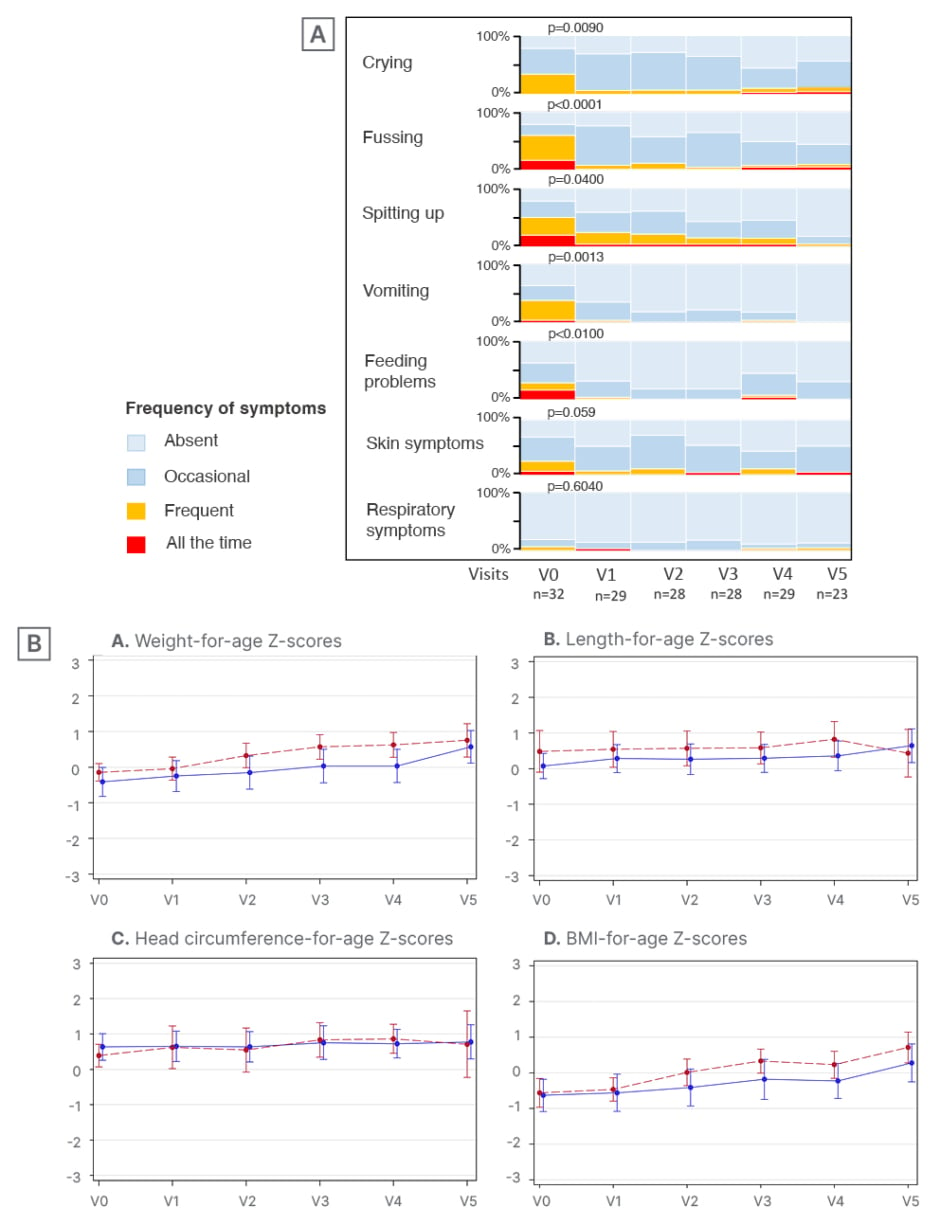
Figure 2: The Platypus study: symptom resolution (A) and growth parameters (B) in infants with moderate-to-severe cow’s milk protein allergy fed human milk oligosaccharide-supplement amino acid formula.29
V: visit.
As with the CINNAMON study, faecal microbiome analysis was also performed as part of the Platypus trial. Results showed an enrichment of the genus Bifidobacterium, with significant changes seen as early as the first month, and maintained to 12 months of age.29 Conversely, there was a significant reduction in potentially pathogenic Proteobacteria, which is a known marker phylum of gut dysbiosis.29 An early enrichment in the four HMO-utilising, infant-type Bifidobacteria was also observed.29 These beneficial bacteria were not detected at baseline, but became more abundant within the first month of feeding with HMO-supplemented formula. Similar to the CINNAMON study, HMO-driven microbiome changes in infants in the Platypus study were also associated with a significant rise in faecal SCFA concentrations. Acetate was the predominant SCFA, and high levels were maintained throughout the study period. Butyrate levels also increased significantly from the second half of the first year of life, and peaked at 12 months of age.29 Collectively, these findings suggest that supplementation of the AAF with 2’FL and LNnT was associated with a significant enrichment in HMO-utilising Bifidobacteria and a partial correction of the gut microbial dysbiosis in infants with CMPA, Nowak-Węgrzyn concluded.
Summarising, Nowak-Węgrzyn reiterated that the microbiome plays a crucial role in steering the development of the early infant immune system. Lactose and HMOs support the developing immune system, and so these nutrients should be considered for non-breastfed infants, Nowak-Węgrzyn stressed. CMPA is an immune-mediated disease, and 2’FL and LNnT play a protective role against allergic disease through several actions. W-EHF with 2’FL and LNnT has been shown to be well-tolerated in infants with CMPA, and appears protective against several infections, possibly also reducing medication use. The impact on the microbiota is to slow microbial diversity in infants with CMPA and enrich for ‘early’ type FCT, similar to that seen in breastfed infants. In the Platypus study, infants with moderate-to-severe CMPA fed an AAF with 2’FL and LNnT, achieved normal growth, with some catch-up growth, and the formula was well tolerated. Notably, supplementation with 2’FL and LNnT was also associated with a significant enrichment in HMO-utilising Bifidobacteria, and a partial correction of the gut microbial dysbiosis in infants with moderate-severe CMPA.
Question and Answer Session
The symposium concluded with an interactive question and answer session with questions posed by the audience. When asked about the possible mechanisms by which HMOs decrease risk of respiratory infections, O’Mahony explained that we do not fully know, but pinpointed metabolites as key in enhancing not just effector components of the immune response to infection, but also the regulatory networks that prevent an overreactive response to infection. These metabolites are absorbed systemically, and can have effects on distant organs such as the lungs, O’Mahony explained.
On the question of which is better for CMPA management, symbiotics or HMOs, Nowak-Węgrzyn acknowledged that there is no simple answer in the absence of direct head-to-head comparisons. However, Nowak-Węgrzyn stressed that 2’FL and LNnT “make a lot of sense” because they are identical to human breast milk HMOs, and are also found at similar concentrations. HMOs in formula are therefore very beneficial and appropriate for use in any infant population, Nowak-Węgrzyn noted.
O’Mahony described faecal samples as the best sample type currently available when questioned on the correlation between levels of SCFA in stool with activity in organs and systems. Clear correlations have been seen between SCFA in faecal samples and allergy protective effects in animal models, O’Mahony noted.
In response to a question on the clinical relevance of the significant on-treatment enrichment of HMO-utilising Bifidobacteria seen in the Platypus study, Nowak-Węgrzyn explained that this shows the supplemented formula is acting in a similar way to breast milk, by enriching the beneficial Bifidobacteria and modulating the gut microbiota in the desired direction.
A question from the Chair to all the panel focused on whether the slower maturation of microbiome diversity with HMOs is beneficial. Nowak-Węgrzyn replied that “more is not always better,” and the dominance of Bifidobacteria and decreased overall diversity seen with HMO-supplemented formula creates a gut environment that closely mirrors that of exclusively breastfed infants, which is beneficial. In contrast, the microbiome of infants fed formula without HMO supplementation evolves differently in the first year, acquiring more adult-type bacteria sooner. Boulangé agreed, highlighting that the timing of metabolite production is also key. Bifidobacteria at the early stages of life are important for ongoing development of the microbiota and create a niche for other bacteria, Boulangé explained. O’Mahony added that it was important to start “gently”, supporting just one or two evolutionally appropriate and beneficial microbes in the newborn infant in order to allow the immune system to develop properly.
The final question asked at what stage of immune development HMOs are most important for the prevention of allergy or beneficial to rebalance the immune response. Nowak-Węgrzyn stressed that the first few months of life are critical, when there is no complementary feeding, and breast milk or formula are the only prebiotic sources. Studies have shown that the infection prevention effect is stronger in healthy infants fed HMO-supplemented formula since birth versus infants with CMPA who typically start treatment later. Timing is critical, and those first few months are most important to set the stage for what comes later, Nowak-Węgrzyn explained, and this also corresponds to when HMOs levels in breast milk are at their highest. “So nature gives us the clues,” she concluded, “you have to set up the microbiome in the gut early.”






