Meeting Summary
Cow’s milk allergy (CMA) is one of the most common infant food allergies, and timely and accurate diagnosis is important to minimise impact on the child’s future health, development, and quality of life. During this symposium, leading experts in paediatric gastroenterology, allergy, and nutrition considered the challenges of both over- and underdiagnosis of CMA, and the key role of the Cow’s Milk-related Symptom Score (CoMiSSTM, Nestlé Health Science, Vers-chez-les-Blancs, Switzerland) in helping to raise awareness of symptoms and support diagnosis. Carina Venter, Professor of Allergy and Clinical Immunology at the University of Colorado, Denver, USA, highlighted potential pitfalls in the diagnosis of CMA, and explained how misdiagnosis can carry consequences for both infants and their families. Yvan Vandenplas, Professor Emeritus at the KidZ Health Castle, University Hospital Brussels, Belgium, outlined the evolution and updates to CoMiSSTM over time, based on increasingly extensive clinical evidence, culminating in the 2022 update to the awareness tool. Hania Szajewska, Professor and Chair of the Department of Paediatrics, Medical University of Warsaw, Poland, presented new data for CoMiSSTM in presumed healthy infants aged between 6–12 months, and explained how the evidence base is continuing to build, strengthening the use and applicability of CoMiSSTM in everyday clinical practice.
Over- or Underdiagnosis of Cow’s Milk Allergy: How Do We Get It Right?
Carina Venter
Venter explained that CMA is by far the most common and complex presentation of food allergy, particularly in early childhood, with the vast majority of cases falling under the umbrella of non-IgE-mediated food allergy.1
CMA symptoms can be IgE-mediated, affecting the skin (acute urticaria and angioedema), the gastrointestinal (GI) system (colicky abdominal pain and diarrhoea), and the respiratory tract (upper and lower respiratory symptoms and anaphylaxis).2 Non-IgE-mediated CMA is also associated with skin symptoms, notably atopic dermatitis, and GI effects, such as gastro-oesophageal reflux, blood and/or mucus in the stools, and faltering growth in severe cases.2 Venter described some symptoms as “questionable” in their association with CMA, but stressed the importance of listening to every family, and taking their concerns into account.
While CMA is undoubtedly one of the most common food allergies seen in infants and young children, the reported prevalence varies considerably worldwide.3-13 Currently, the overall global prevalence of CMA is estimated to lie between 1–3%.14 A recent European systematic review and meta-analysis of 93 studies concluded that the point prevalence of self-reported specific CMA was 5.7%, while the point prevalence based on oral food challenge (OFC) was 0.3%.15 To illustrate potential pitfalls in diagnosis, Venter presented data from a study she conducted in 969 children born on the Isle of Wight, UK, between 2001–2002.12 Of the 26 infants diagnosed with CMA, 92% had a mild-moderate non-IgE-mediated form based on double-blind placebo-controlled food challenges (DBPCFC).12 Although only approximately 3% of the total cohort were ultimately diagnosed with challenge-proven food allergy, over 50% had food-related symptoms reported by their parents.12,16 All the children with a negative DBPCFC following a positive OFC also showed subjective symptoms during the open food challenge, highlighting the need for reintroduction.16
The list of International Milk Allergy in Primary Care (iMAP) symptoms potentially related to non-IgE-mediated food allergy is extensive, and Venter cautioned that no clinician should diagnose on this basis alone.17 In a secondary analysis of 1,303 EAT study participants aged 3–12 months assessed against iMAP guidelines, one in 11 infants was deemed to have two or more severe iMAP symptoms, while three-quarters had two or more mild/moderate symptoms.18 Aside from presenting symptoms, clinicians therefore need to ask other important questions when diagnosing CMA. These include issues such as family history of atopic disease; sources and intake of cow’s milk; concerns with feeding and/or poor growth; changes in diet; and other management approaches, including medication.19 Many different CMA symptom scores exist, with results from a recent systematic review identifying 10 online questionnaires.20 However, of these available symptom scores, only the CoMiSSTM tool has been clinically validated, Venter emphasised.
Despite the differences and sometimes overlapping manifestations between the different forms of non-IgE-mediated food allergies, the diagnostic process is uniform. There is no conclusive test to confirm or exclude CMA; therefore, the diagnosis must be based on the history of clinical manifestations upon consumption of the offending food. The diagnostic process starts with a period (usually 2–4 weeks) of elimination of cow’s milk from the diet. If the symptoms disappear during food exclusion, the allergenic food needs to be reintroduced by OFC or home reintroduction to confirm the diagnosis. Venter stressed that this food reintroduction step is absolutely crucial, in order to make a clear diagnosis of CMA.
The dangers of under- or delayed diagnosis of CMA underscore the importance of obtaining a timely and accurate diagnosis. These include a detrimental impact on the child’s health, such as faltering growth, an increased risk of micronutrient deficiencies, poor sleep, and feeding difficulties.21,22 Delays or underdiagnosis of CMA can also adversely affect the family and the wider healthcare system.21-24 Equally, overdiagnosis of CMA also carries potential adverse consequences, with infants who avoid a food due to a perceived allergy found to experience the same negative effects on quality of life as those with a diagnosed allergy.25 Avoidance of cow’s milk can have both short- and long-term implications, leading to less overall dietary diversity, and a retained preference for bitter tastes, resulting in reduced dairy consumption.26
In conclusion, Venter reiterated that CMA is one of the most common and complex food allergies of early childhood. The diagnosis of CMA is challenging, particularly for non-IgE-mediated CMA, and both under- and overdiagnosis can have consequences. Ultimately, it is important to support families in order to reach an accurate diagnosis as soon as possible, she concluded.
What is the Role of CoMiSSTM in Preventing Over- or Underdiagnosis?
Yvan Vandenplas
CMA is defined as a reproducible adverse reaction to one or more milk proteins (usually casein or whey β-lactoglobulin) mediated by IgE and/or non-IgE mechanisms.27 The prevalence of CMA in the first year of life ranges from 0.5–3.0%, and there is considerable variability in the international data.14,28,29 Knowing the true prevalence of CMA, and exactly how many infants suffer with this allergy, therefore remains challenging, Vandenplas acknowledged. In terms of the frequency of CMA symptoms, up to 60% of infants experience digestive symptoms, 30% respiratory, 60% skin, and 50% general symptoms, such as crying.30 Vandenplas stressed that the vast majority of people with CMA present with a combination of these different organ system symptoms, and less than 1% of infants with non-IgE-mediated CMA present with only one symptom.24
Unlike with IgE-driven food allergy, the real challenge lies in non-IgE-mediated CMA, where there is no definitive laboratory test to aid in diagnosis. A number of online CMA questionnaires are available to parents and healthcare professionals (HCP), but these vary in the symptoms they cover, and most are not validated or evidence-based.20 To address these diagnostic challenges, Vandenplas explained that the CoMiSSTM awareness tool was developed to help HCPs consider a possible CMA diagnosis in a child presenting with a mix of mild-to-moderate symptoms. To date, over 20 original studies, many of them randomised controlled trials, have been conducted using CoMiSSTM.31
Vandenplas went on to review the key symptoms that are scored in CoMiSSTM.32 Regurgitation and crying are assigned a score from 0–6, according to severity. Stools are given a score of 0, 4, or 6 based on the Brussels Infants and Toddlers Stool Scale (BITSS), which is used to evaluate the consistency of stools in infants less than 1 year of age. GI symptoms and stool changes should only be considered if they last ≥1 week and occur in the absence of infectious disease. Skin manifestations that are scored in CoMiSSTM include atopic eczema and (acute) urticaria and/or angioedema. Atopic eczema should only be considered if duration is ≥1 week, and is assigned a simplified scale score of 0–6 based on an estimation of the surface covered by dermatitis. Urticaria/angioedema is assigned an absolute score of 0 (No) or 6 (Yes), because if directly related to cow’s milk ingestion, this is strongly suggestive of CMA. Finally, respiratory symptoms are also considered in CoMiSSTM, but do not carry the same weight as other symptoms, as they are most often caused by viral infections in infants. Respiratory symptoms encompass chronic cough, runny nose, and wheezing, and are assigned a score of 0–3 based on severity. The result is a final CoMiSSTM score ranging from 0–33, which can be interpreted as follows:32
• Total score ≥10: may be suggestive of cow’s milk-related symptoms, and could potentially be CMA.
• Total score <6: symptoms are not likely to be related to CMA. Look for other causes.
Vandenplas confirmed that the CoMiSSTM scoring form is not intended to be used as a diagnostic tool, and should not replace an OFC.31 CMA diagnosis should be confirmed by a 2–4 week elimination diet, followed by an OFC.31
In 2023, the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) published a position paper on the diagnosis, management, and prevention of CMA.33 As one of its 73 consensus statements, this paper specifies that the baseline CoMiSSTM and its reduction during an elimination diet may be indicative for CMA, but is not diagnostic.33 So, while CoMiSSTM might increase awareness, and thus favour overdiagnosis, it might also decrease overdiagnosis, since symptoms in at least two organ systems are needed for a score ≥10, Vandenplas explained. It also avoids the unnecessary use of elimination diets in children presenting with one single manifestation who are considered as having CMA, he added. Data from the UK indicated that increasing awareness of CMA was associated with an increase in the prescription rate of hydrolysed formula. However, this was accompanied by a decrease in the prescription of medication, which Vandenplas described as “inappropriate” in many cases, and potentially associated with adverse events.34
The Chinese prospective, multicentre study, MOSAIC, illustrated that infants placed on an elimination diet showed a significant reduction in the CoMiSSTM score for both skin and respiratory symptoms, as well as reduction in total CoMiSSTM score (Figure 1).35 The authors concluded that although CoMiSSTM cannot be considered a standalone CMA diagnostic tool, it nonetheless represents a useful awareness tool for the early identification of infants with CMA-related symptoms, as well as for monitoring symptom improvement.35 A decrease of CoMiSSTM score during a cow’s milk elimination diet has been shown to be predictive of a reaction to the OFC.31 The CoMiSSTM tool was updated in 2022 and, based on increasing numbers of studies and reanalysis of the literature, the panel concluded that the cut-off indicating the likelihood that symptoms may be cow’s milk-related should be lowered from ≥12 to ≥10, and that CMA is unlikely if CoMiSS is ≤6.36
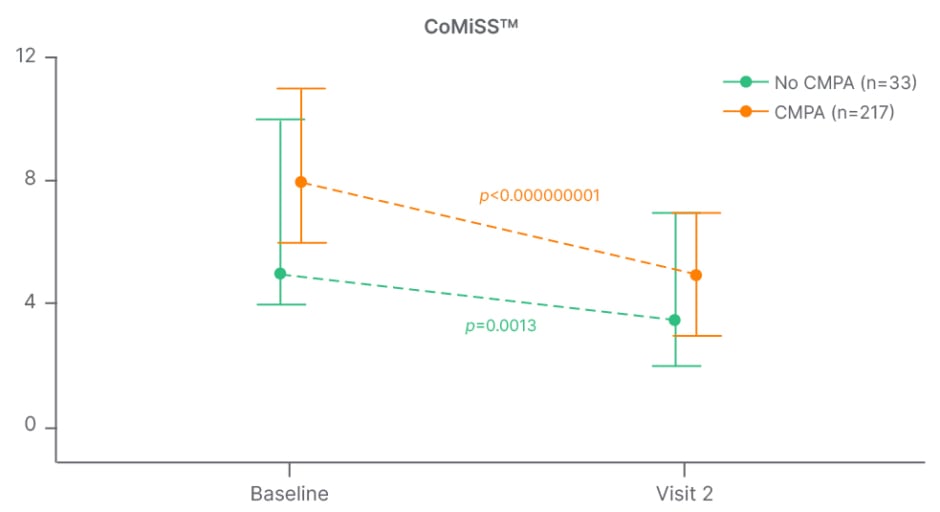
Figure 1: MOSAIC study: change in CoMiSSTM score from baseline to visit 2 in infants with versus without cow’s milk protein allergy.35
CMPA: cow’s milk protein allergy; CoMiSSTM: Cow’s Milk-related Symptom Score.
The key principles of CMA diagnosis remain a cow’s milk protein elimination diet for 2–4 weeks, followed by the reintroduction of cow’s milk through a food challenge. According to the ESPGHAN consensus paper, in clinical practice, the open OFC is clinically more feasible and practical than DBPCFC, and is sufficient to confirm the diagnosis of CMA, and the development of oral tolerance.33 In IgE-mediated CMA, the OFC test should be supervised by trained medical HCPs.33
In summary, Vandenplas confirmed that CMA is a complex and increasingly common disorder, which is difficult to diagnose. Its reported prevalence ranges from 0.5–3.0% depending on the region, with most infants outgrowing their non-IgE-mediated allergy in early childhood. IgE-mediated CMA is usually rapid in onset and relatively easy to diagnose with tools available to support the diagnosis, while non-IgE-mediated CMA can be delayed and dose-dependent, and is difficult to diagnose given the lack of supportive tools. Lack of symptom awareness can also lead to CMA being misdiagnosed, with both over- and underdiagnosis having consequences for children. CoMiSSTM, which was updated in 2022, was designed to support the diagnosis of CMA, and to raise awareness. It remains a simple, easy, fast, non-invasive awareness tool, used to support the diagnosis of CMA in infants, and can be effective in helping HCPs avoid the pitfalls of both under- and overdiagnosis (Figure 2).

Figure 2: CoMiSSTM: Cow’s Milk-related Symptom Score.
CMA: cow’s milk allergy.
Trademark of Société des Produits Nestle S.A., Vevey Switzerland © 2022 Nestlé.
All rights reserved.
CoMiSSTM Data in Presumed Healthy Infants: How Does this Strengthen our Clinical Practice?
Hania Szajewska
An editorial on the 2023 European Academy of Allergy and Clinical Immunology (EAACI) guidelines on the diagnosis of IgE-mediated food allergy states: “Infants and young children are particularly vulnerable from a nutritional standpoint, and so it is vital to ensure that a robust diagnosis is made in a timely manner to mitigate any effects on growth and avoid the development of aversive eating behaviours.”37 The challenges of recognising CMA in infants are well known, particularly non-IgE-mediated allergy, and Szajewska reaffirmed that CoMiSSTM is a useful awareness tool for evaluating symptoms related to cow’s milk. However, while CoMiSSTM values are already available for healthy and symptomatic infants aged 0–6 months, data for CoMiSS is still needed in presumed healthy infants between 6–12 months old.
In order to plug these data gaps and strengthen the application of the CoMiSSTM tool in clinical practice, a study was undertaken to determine CoMiSSTM values in presumed healthy infants between 6–12 months old.38 Importantly, this represents the age range where the diagnosis of CMA is often established. The international, multicentre, cross-sectional study was conducted between September 2022–August 2023, and enrolled presumed healthy infants aged 6–12 months attending well-child clinics. Exclusion criteria were preterm delivery, acute/chronic disease, and the use of therapeutic formula, dietary supplements, and medication. CoMiSSTM assessments were carried out via questionnaires completed by the HCP, with interviews conducted in native languages. In total, 609 healthy European infants aged 6–12 months were included from Poland, Spain, Italy, Bulgaria, Czechia, and Belgium.38
Szajewska presented results from this study, which were recently published, showing the CoMiSSTM score in healthy European infants aged 6–12 months across different percentiles (Table 1).38 The median CoMiSSTM value was 3, which is in line with that seen in the younger population up to 6 months of age, she explained. The median CoMiSSTM was highest in infants at 6 months of age (4) and lowest at 12 months of age (1). When looking at the distribution of CoMiSSTM score according to age, significant differences were observed across age groups, notably between infants aged 6 months versus 10 months (p=0.001), and infants aged 6 months versus 12 months (p=0.007). The study also found no differences in CoMiSSTM scores according to sex (p=0.55) or feeding type (exclusive breastfeeding versus not exclusive breastfeeding; p=0.9). Similarly, for individual CoMiSSTM symptoms, no significant differences were seen according to gender or feeding type. However, significant differences were noted for symptoms of regurgitation (p=0.02) and stools (p<0.001), when analysed by infant age.38
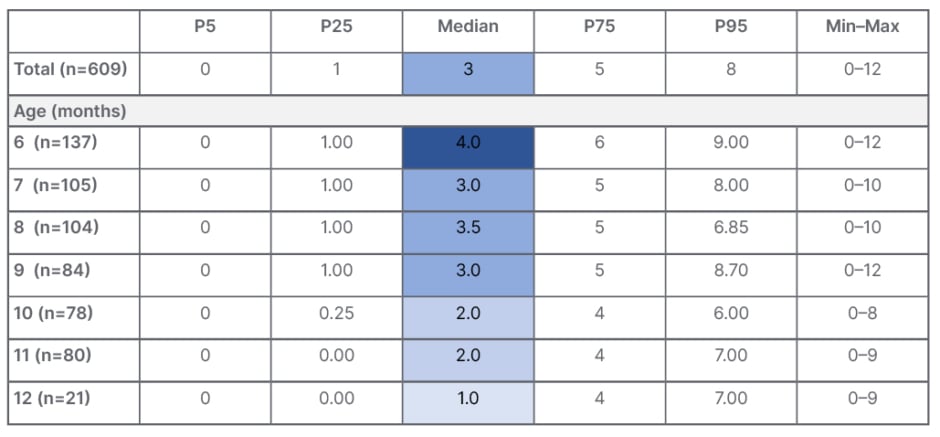
Table 1: CoMiSSTM in healthy European infants aged 6–12 months.38
CoMiSSTM: Cow’s Milk-related Symptom Score; P: percentile.
Overall, this cross-sectional study provides CoMiSSTM values for presumed healthy European infants aged 6–12 months, extending understanding beyond the commonly studied age range (0–6 months), Szajewska concluded. Potential study limitations included the use of convenience sampling for pragmatic reasons, the cross-sectional study design, and uneven cohort sizes from different countries. Further research is also needed to assess the use of CoMiSSTM as an awareness tool for cow’s milk-related symptoms for symptomatic infants older than 6 months. As such, the planned next step consists of a cross-sectional, multicentre study to determine CoMiSS™ values in infants aged 6–12 months, with symptoms suggestive of CMA or functional GI disorders. Infants with prior acute or chronic disease, or on current therapeutic formula treatment, will be excluded. CoMiSSTM assessment will be performed in the same manner as in the prior study of healthy infants.
Szajewska conceded that some may question whether these awareness tools for allergic diseases are needed at all. Possible reasons why they may not be include the risk of misdiagnosis due to reliance on self-diagnosis, the potential induction of anxiety or unnecessary concern through over-emphasis on allergy symptoms, and the medicalisation of normal symptoms, where heightened awareness causes people to seek treatment for normal reactions or minor issues. Nonetheless, Szajewska insisted that there are several important reasons why awareness tools such as CoMiSSTM are needed, and for CMA in particular. One, awareness and education help the public to understand symptoms, thereby aiding early diagnosis. Two, as allergic symptoms often mimic and overlap with those of other conditions, awareness tools can help pinpoint those with true CMA. Finally, awareness tools can contribute to efficient consultations as, when patients are more informed, they can communicate their symptoms and concerns more effectively. Similarly, when primary care HCPs are better informed, they will refer patients in a more judicious way, potentially saving time and resources, Szajewska added.
The compromise therefore lies in a balanced approach to using awareness tools like CoMiSSTM, focused on user education. For optimal health management, the awareness tool should be integrated with established diagnostic procedures, in order to help ensure a robust approach. As her key take-home message, Szajewska pointed to the statement on awareness and management tools from the 2023 ESPGHAN position paper: “The baseline CoMiSSTM and its reduction during an elimination diet may be indicative for CMA, but is not diagnostic.”33
Live Q&A
All Panel
The symposium presentations were followed by a question-and-answer session, in which the speakers responded to questions posed by the audience and other panel members.
A paediatric dietitian in the audience commented that it was “interesting” to see respiratory symptoms included in the CoMiSSTM tool, as this aligns with experience from a recent cohort study, which found that approximately 25% of infants with CMA had respiratory symptoms. She also agreed with the inclusion of constipation, describing this as a key CMA symptom which is often overlooked, but suggested that soft stool constipation and straining should be considered, alongside stool appearance. The CoMiSSTM tool has considerable value, the audience member concluded, but it is important to ensure the skills and knowledge are in place to enable it to be used effectively to support diagnosis, particularly among HCPs involved in first-line CMA management.
In response to this audience comment, Venter agreed that it can be a “difficult balance” between over- and underdiagnosis of CMA, and highlighted the value of collaboration between dietitians, allergists, gastroenterologists, and paediatricians. This multidisciplinary approach is particularly important in CMA because of the huge nutritional impact, she stressed.
In response to a question on the potential use of CoMiSSTM as an awareness tool for other food allergies, Vandenplas said this would require further testing and study because, until now, CoMiSSTM has only been validated in CMA.
Vandenplas was also asked whether the CoMiSSTM tool can be used to assess the severity of CMA. “Yes and no,” he replied, as the higher the score, the more predominant the symptoms are, and therefore, the more the infant will suffer. However, Vandenplas went on to explain that some of the most severe symptoms of CMA, such as anaphylaxis, food protein-induced enterocolitis, and blood in the stools, were deliberately excluded from CoMiSSTM. This is because these symptoms warrant immediate action by an HCP, and/or have a broad differential diagnosis that requires careful consideration, he noted.
A final practical question from the audience on non-IgE-mediated CMA asked how parents can be encouraged to reintroduce milk after 2–4 weeks if their infant’s symptoms have improved, and they are understandably reluctant to do so. Venter said it was important to explain to parents beforehand that, based on the data, approximately 50% of babies will be expected to improve on avoidance, and symptoms will not recur on reintroduction. The reasons for this are unclear, but may be due to maturation of the infant’s digestive system. Some HCPs sign a contract with the family stipulating that they agree to reintroduction. However, Venter acknowledged that, in clinical practice, “we are working with human beings, and not doing mouse model experiments,” so there is ultimately nothing that can be done if a family refuses reintroduction. In this case, she suggested that a shorter follow-up time (1 month rather than the usual 3–6 months) may be prudent to either revisit reintroduction or potentially discuss starting the infant on the Milk Ladder.





