BACKGROUND
MOv18 IgE is a first-in-class IgE antibody targeting FRα, an antigen overexpressed in ovarian and other cancers.1 IgE-based anti-cancer antibodies offer significant advantages, such as the ability to bind strongly to immune effector cells via the FcɛRI receptor and to stimulate tumour-infiltrating immune cells against cancer. In a recent Phase I clinical trial, MOv18 IgE demonstrated promising efficacy, but also triggered transient urticarial skin reactions. The authors aimed to elucidate the immunological basis of these reactions and assess whether they were mediated by allergic mechanisms.
METHODS
Twenty-four patients with FRα-expressing tumours were treated with escalating doses of MOv18 IgE. Clinical data regarding urticarial skin reactions were collected. Immunohistochemistry was conducted to evaluate FRα expression in human skin, and immuno-mass spectrometry and transcriptomic analyses were performed on urticarial and non-involved skin biopsies from a patient who received the highest antibody dose. These analyses evaluated immune cell infiltration and mast cell degranulation in the skin. Circulating immunological markers, including β-tryptase levels and basophil activation, were assessed to investigate any association with a systemic allergic response.
RESULTS
Urticarial skin reactions occurred in 62.5% of patients, with most reactions being mild and diminishing over repeated doses (Figure 1). These reactions were not associated with serum β-tryptase elevation, indicating an absence of systemic allergic activity. Immunohistochemistry and immuno-mass spectrometry analyses confirmed that FRα was not expressed in normal skin, and MOv18 IgE did not bind to skin antigens. Lesional skin biopsies from a patient who developed a urticarial reaction showed scattered eosinophils and neutrophils, along with mast cell degranulation, but no increased immune cell infiltration. Transcriptomic analysis revealed activation of pro-inflammatory, but not of allergic, pathways. No cytokine markers of allergic hypersensitivity and no basophil activation were detected in the patient’s circulation.
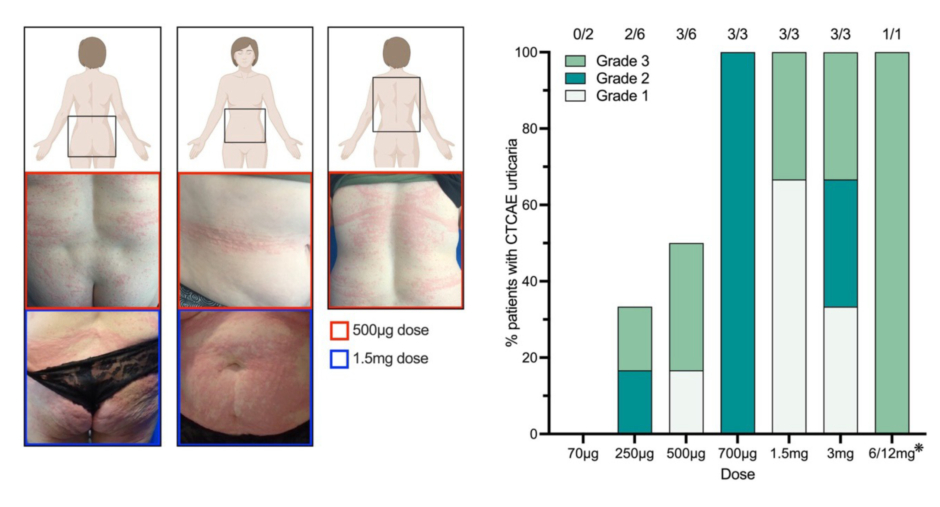
Figure 1: Readily manageable urticarial adverse events were associated with higher doses of MOv18 IgE treatment.
Left Panel: Representative images of urticarial skin reactions, seen in a patient who received a 500 μg dose (orange) and a patient who received a 1.5 mg dose (blue) from the larger cohort of patients treated with MOv18 IgE.
Right panel: Proportion and CTCAE urticarial grade per dosing cohort of patients treated with MOv18 IgE (urticarial: n=15; total N=24). *Intra-patient dose escalation was performed with this patient (n=1), who received three doses at 6 mg, followed by three doses at 12 mg.
Adapted from Stavraka C et al.1
CTCAE: Common Terminology Criteria for Adverse Events.
CONCLUSION
The urticarial skin reactions observed in patients treated with MOv18 IgE were not driven by allergic mechanisms or by skin antigen recognition by the antibody. Instead, these reactions likely represent infusion-related phenomena commonly seen with monoclonal antibody therapies. The authors’ findings support the overall safety of MOv18 IgE, with the reactions being manageable. These data are crucial for advancing IgE antibodies in cancer immunotherapy while ensuring patient safety.




