Interview Summary
Angioedema (AE) syndromes are associated with recurrent, unpredictable attacks of subcutaneous and submucosal tissue swelling, which can become life-threatening. Recent classification efforts define a group of rare forms of AE mediated by overproduction of the vasodilator bradykinin. To discuss the role of bradykinin in AE, the EMJ interviewed AE specialist Danny M. Cohn, University of Amsterdam, the Netherlands. Cohn described the manifestations (attacks), pathogenesis, and diagnosis of bradykinin-mediated forms of AE, and considered the unmet treatment needs in these patients. The potential for new therapies targeting the bradykinin B2 receptor was discussed, alongside recent data for bradykinin B2 receptor antagonism.INTRODUCTION
AE is a clinical manifestation in which a temporary increase in vascular permeability causes excess fluid to leak from the blood vessels and accumulate in the subcutaneous and/or submucosal tissues, causing intermittent, localised, self-limiting swelling which can be painful and debilitating.1-3
Patients with recurrent forms of AE experience attacks of AE that can be unpredictable.2 Swelling can affect visible areas such as the limbs and face, but also internal regions of the body, such as the gastrointestinal tract or upper airways, where it can become life-threatening.2 AE attacks can often be debilitating for patients, interfering with their normal daily activities, and fear of attacks can also cause depression and anxiety.2
Although gaps remain in our knowledge and understanding of AE forms, classifying types and sub-types by their underlying mechanisms, as in the recently published Definition, Acronyms, Nomenclature, and Classification of angioEdema (DANCE) consensus, may lead to more accurate diagnosis and targeted treatment.1 The most common form of AE is mast-cell mediated (AE-MC), such as AE associated with urticaria.1 Other types of AE are grouped together into bradykinin-mediated AE (AE-BK), vascular-endothelium dysfunction AE (AE-VK), drug-induced AE (AE-DI), or AE with unknown aetiology (AE-UNK; Figure 1).1
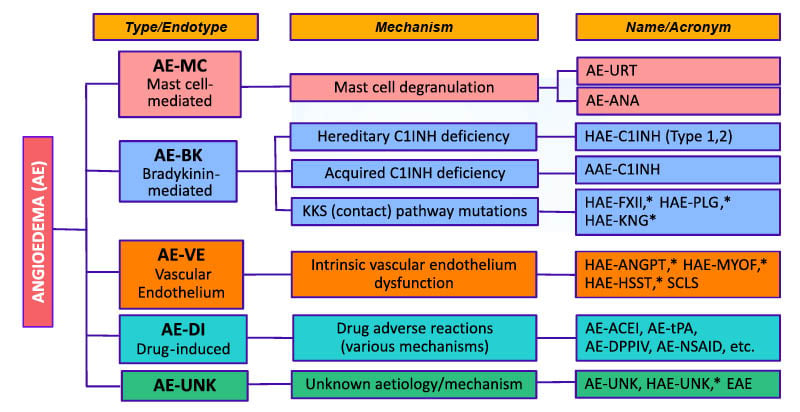
Figure 1: New classification of angioedema syndromes.
Figure reproduced from Reshef et al.1 2024.
*Also designated: Normal C1INH angioedema (HAE-nC1INH).
AAE: aquired AE; AAE-C1INH: acquired AE due to C1INH deficiency; AE: angioedema; AE-ACEI: AE due to angiotensin-converting enzyme inhibitors; AE-ANA: AE due to anaphylaxis; AE-BK: Bradykinin-mediated AE; AE-DI: drug-induced AE; AE-DPPIV: AE due to di-peptidyl peptidase IV blockers; AE-MC: mast cell–mediated AE; AE-NSAID: AE due to nonsteroidal anti-inflammatory drugs; AE-tPA: AE due to tissue-type plasminogen activators; AE-UNK: AE due to unknown cause; AE-URT: AE due to urticaria; AE-VE: AE due to vascular endothelium dysfunction; C1INH: C1 inhibitor; EAE: episodic AE with eosinophilia; HAE: hereditary AE; HAE-ANGPT: HAE due to angiopoietin 1 mutation; HAE-C1INH: HAE due to C1INH deficiency; HAE-FXII: HAE due to factor XII mutation; HAE-HSST: HAE due to heparan sulfate 3-O-sulfotransferase 6 mutation; HAE-KNG: HAE due to kininogen 1 mutation; HAE-MYOF: HAE due to myoferlin mutation; HAE-PLG: HAE due to plasminogen mutation; HAE-UNK: HAE due to unknown cause; KKS: kallikrein-kinin system; SCLS: systemic capillary leak syndrome
In some cases, AE has a genetic basis and can be hereditary, and in other cases, AE can be acquired due to underlying disease; several of these subtypes are bradykinin-mediated and are grouped together as AE-BK.1
PATHOGENESIS OF BRADYKININ-MEDIATED ANGIOEDEMA
The DANCE consensus defines three subtypes of AE-BK: hereditary C1 inhibitor deficiency (HAE-C1INH), acquired C1 inhibitor deficiency (AAE-C1INH), and kallikrein-kinin (contact) pathway mutations which are also designated as forms of hereditary AE with normal C1 inhibitor (HAE-nC1INH).1 C1 inhibitor functions as a major regulator of several proteases in the complement, contact system, coagulation, and fibrinolytic pathways. A deficiency in functional C1 inhibitor leads to dysregulated activity of activated factor XII and plasma kallikrein, resulting in an overproduction of the potent vasodilator, bradykinin.2 A small peptide, bradykinin binds to the bradykinin B2 receptor on blood vessel walls leading to increased vascular permeability and localised swelling (Figure 2).3
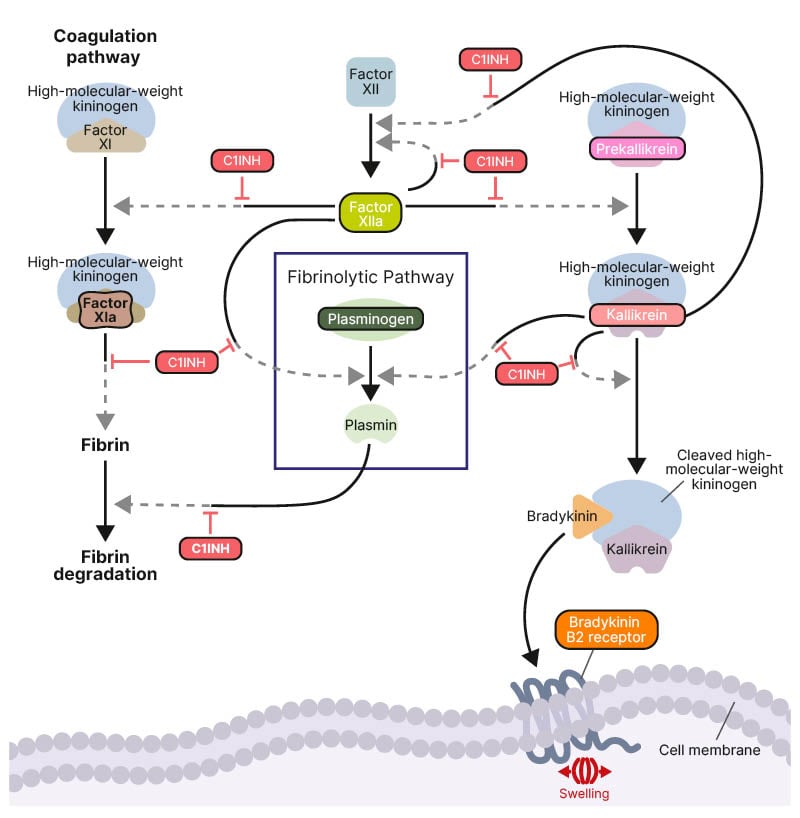
Figure 2: Molecular pathways in hereditary angioedema pathophysiology.
Figure reproduced from Riedl et al.2 2024.
Activation is indicated by solid black arrows.
C1INH: C1 inhibitor.
Cohn explained that both HAE-C1INH and AAE-C1INH involve the excessive release of bradykinin due to lack of control by C1 inhibitor. HAE-C1INH is the most common form of AE-BK, estimated to affect approximately 1 in 50,000 individuals globally, and is caused by an inherited mutation in the SERPING1 gene that encodes C1 inhibitor.3
AAE-C1INH, estimated to affect approximately 1 in 500,000 individuals,4 typically occurs later in life, and is associated with underlying conditions such as lymphoproliferative disorders, plasma cell dyscrasias (e.g., monoclonal gammopathy), or autoimmune diseases.5,6 AAE-C1INH is caused by either excessive consumption of C1 inhibitor or neutralisation of C1 inhibitor by autoantibodies.3,7
The AE-BK subtype that consists of mutations affecting factor XII, plasminogen, or kininogen-1 are also very rare. These mutations are designated forms of HAE-nC1INH.3 Cohn noted that “[these] forms of HAE-nC1INH are believed to be bradykinin-mediated, and mutations affect the bradykinin-forming cascade pathway.”3
DIAGNOSIS OF BRADYKININ-MEDIATED ANGIOEDEMA
AE-BK should be suspected when a patient presents with a history of recurrent skin swelling, abdominal pain, and/or swelling in the larynx.3 Diagnosis of HAE-C1INH or AAE-C1INH is performed by also measuring the levels and activity of C1 inhibitor, and the levels of C4, in the blood.3
Although complement C4 and C1 inhibitor activity are decreased in both HAE-C1INH and AAE-C1INH, the latter can be differentiated by later age of onset, underlying diseases, and often decreased C1q, a component of the complement system.3 Genetic analysis can support differential diagnosis, as pathogenic mutations in the SERPING1 gene are found in about 90% of patients with HAE-C1INH.8 Cohn explained: “Genetic analysis can confirm the diagnosis of HAE and may be used for cascade screening.”
Diagnosis of the AE-BK forms of HAE-nC1INH depends on clinical history, genetic testing, and therapeutic trials.9 Using laboratory tests, HAE-nC1INH can currently only be diagnosed by genetic testing, and in patients suspected to have AE-BK but with normal C1 inhibitor levels, guidelines recommend testing for mutations in genes that encode factor XII gene, plasminogen, and kininogen-1.3
UNMET TREATMENT NEEDS IN BRADYKININ-MEDIATED ANGIOEDEMA
Treatment options for HAE-C1INH are divided into on-demand and prophylactic therapy.3 Guidelines recommend the use of intravenous C1 inhibitor concentrates, ecallantide (a plasma kallikrein inhibitor), or icatibant (a competitive antagonist of the bradykinin B2 receptor) as on-demand therapy, although approved product indications vary between countries.3 Recommended prophylactic therapies for HAE-C1INH include subcutaneous C1 inhibitor concentrates, lanadelumab (a plasma kallikrein inhibitor), or berotralstat (a plasma kallikrein inhibitor).3
Among patients with HAE-C1INH, Cohn explained that one of the main unmet needs is an effective, well-tolerated, oral treatment option. “There are many challenges that these patients face which leads to them forgo or postpone their treatment,” he said, “[including] the difficulty in finding a quiet place to inject or finding the time to administer, a fear of needles, or dependence on healthcare providers or caregivers to administer the drug.” He also emphasised that subcutaneous administration can be quite painful, which can be an issue for patients who need to self-administer their treatment. “We know that the sooner you treat [an AE attack], the less severe the attack will become, and the sooner you will recover from it,” he said. “[Providing patients with] more options is always beneficial.”
Cohn pointed out that “there is far more evidence to support recommendations for the treatment of HAE-C1INH than there is for AAE-C1INH; data for the acquired form tend to be case series [rather than large, controlled studies], because it is so rare.” Indeed, there are currently no approved drugs available for on-demand or prophylactic treatment of AAE-C1INH.7 Among the treatments that are used off-label in AAE-C1INH (those approved for use in HAE-C1INH), C1 inhibitor concentrates are considered to be less effective in patients with AAE-C1INH, likely due to rapid degradation or neutralisation.7
Similarly, due to the rare nature of the form of AE, evidence for the efficacy and safety of drugs from controlled studies are lacking in patients with HAE-nC1INH.
THERAPEUTIC POTENTIAL OF TARGETING THE BRADYKININ B2 RECEPTOR
The interaction between bradykinin and the bradykinin B2 receptor is the final component in the pathogenic pathway that leads to the swelling in AE-BK. Antagonism of the bradykinin B2 receptor has represented an effective and well-tolerated therapeutic approach for on-demand treatment of HAE for more than a decade.10 Cohn emphasised that there is potential for this therapeutic approach to be effective beyond this indication. For example, he has observed that the administration of a subcutaneous bradykinin B2 receptor antagonist is effective in patients with AAE-C1INH in his clinic; although this has not been assessed in a clinical trial.
The small, blinded study, POP-AID,11 was conducted in the Netherlands to assess an oral, competitive antagonist of the bradykinin B2 receptor as both an on-demand (Part 1) and prophylactic (Part 2) treatment in AAE-C1INH.7 Part 1 of the study assessed the efficacy of three single oral dose regimens of the study drug (10 mg, 20 mg, and 30 mg) versus placebo to achieve AE symptomatic relief and resolution in four consecutive AE attacks. Both the patient and medical staff were blinded to the nature of the medication, and the order in which the study drug doses and placebo were administered was randomised. The participants completed several questionnaires at pre-specified time points to record patient-reported outcomes. In Part 2, the efficacy of 20 mg twice daily of the study drug in oral capsule form versus placebo was assessed in a double-blind, crossover design. Three participants were treated for two consecutive treatment periods of 8 weeks with a study drug or placebo in a randomised order. The number of investigator-confirmed AE attacks, as well as patient-reported outcomes and safety data, were recorded.7
Results from Part 1 (on-demand treatment) showed that the mean 3-symptom visual analogue scale (VAS-3; skin pain, skin swelling, and abdominal pain) reduced rapidly from baseline to 4 hours after treatment with all three doses of study drug, whereas an increase in VAS-3 was observed with placebo, requiring rescue medication at 6 hours after treatment. No adverse events occurred during Part 1. In Part 2 (prophylactic treatment), all patients were attack-free during treatment with the study drug, but all experienced AE attacks during their placebo period. In Part 2, seven adverse events occurred with the study drug and six with placebo, none of which were serious. One adverse event was considered to be drug-related (abdominal pain after ingestion of capsules without water) and resolved after reinstruction to use water for ingestion.7
Cohn also explained that all three patients from this study, and one additional patient, were subsequently enrolled in an open-label extension study (ONCE-AID), where after 1 year of treatment, 3/4 participants remain free of AE attacks (one participant experienced an AE attack 2 days after treatment initiation).12
An oral, competitive antagonist of the bradykinin B2 receptor has also been assessed for both on-demand and long-term prophylactic treatment in patients with the more common form of AE-BK, HAE-C1INH.13,14 Cohn confirmed that outcomes for on-demand treatment in the RAPIDe-1 trial were very similar to those observed in the smaller POP-AID study.7,13 In an analysis from the ongoing extension study, the majority of attacks achieved complete attack resolution with the study drug within 24 hours.15,16 He also described the long-term prophylactic treatment outcomes as positive.15,17
CONCLUSION
There are limited options for the treatment of HAE-C1INH.3 In addition, there are no approved treatments for AAE-C1INH , and data from controlled trials for the efficacy and safety of treatments in AAE-C1INH and HAE-nC1INH are lacking.7
Cohn summarised by saying: “We have observed clinically that antagonising the bradykinin B2 receptor is effective in these patients, and that’s why we are focusing on [bradykinin B2 receptor antagonists], both for on-demand and prophylactic treatment.” As the final component in the pathogenic pathway that leads to AE attacks in these forms of AE, targeting the bradykinin B2 receptor is a reasonable and viable mechanism in AE-BK.7,15-17




