BACKGROUND AND AIMS
Chest pain is among the most common reasons for presentation to the emergency department, yet identifying patients at the highest short-term risk remains a clinical challenge.1,2 Conventional triage tools rely on clinical variables and troponin assays, but these have limited sensitivity at the time of arrival.3 Recent advances in deep learning applied to ECGs have demonstrated the ability to detect arrhythmias, structural heart disease, and prevalent atherosclerotic cardiovascular disease, but their application to acute chest pain risk stratification has been less studied.4-6 The authors evaluated whether a convolutional neural network (CNN)-derived ECG risk score could improve the prediction of near-term cardiovascular mortality in patients presenting with chest pain.
MATERIALS AND METHODS
This retrospective cohort study leveraged over 1.5 million ECGs recorded at Stanford, California, USA, between 2005–2019. After exclusions for low-quality tracings, missing troponin, and non-chest pain presentations, the final study cohort comprised 22,590 adults presenting with chest pain. Raw waveform data from 12-lead ECGs were used to train and validate a CNN model to generate a continuous ECG risk score (ECG-Risk). Outcomes were ascertained from electronic health records and the Social Security Death Index (SSDI). The primary endpoint was 30-day all-cause mortality, while secondary endpoints included 365-day mortality and a composite of 30-day cardiovascular mortality or revascularisation (percutaneous coronary intervention or coronary artery bypass grafting). Multivariable logistic regression models incorporated ECG-Risk alongside age, troponin, race, blood pressure, hyperlipidaemia, diabetes, prior myocardial infarction, and smoking status. Model discrimination was assessed with the area under the receiver-operating characteristic curve, and improvements were evaluated with DeLong testing and reclassification indices.
RESULTS
In the overall cohort (median age: 55 years; 52% female), the 30-day mortality rate was 1.4%. ECG-Risk was strongly associated with short-term mortality in both unadjusted analysis (odds ratio [OR]: 3.74; 95% CI: 2.91–4.82) and adjusted models (OR: 3.47; 95% CI: 2.66–4.54). Notably, no 30-day deaths occurred in patients with an ECG-Risk below –1.25, suggesting potential utility as a rule-out threshold. Incorporation of ECG-Risk significantly improved prediction beyond traditional clinical variables and troponin (area under the receiver-operating characteristic curve: 0.80 versus 0.75 for baseline model; p<0.05), and net reclassification analyses confirmed incremental prognostic value (Figure 1). ECG-Risk also predicted secondary endpoints, including higher 365-day mortality (adjusted OR: 2.03; 95% CI: 1.80–2.30) and the 30-day mortality/revascularisation composite (adjusted OR: 2.43; 95% CI: 2.17–2.72). Importantly, ECG-Risk was not correlated with troponin levels (p>0.05), highlighting its ability to capture independent prognostic information.
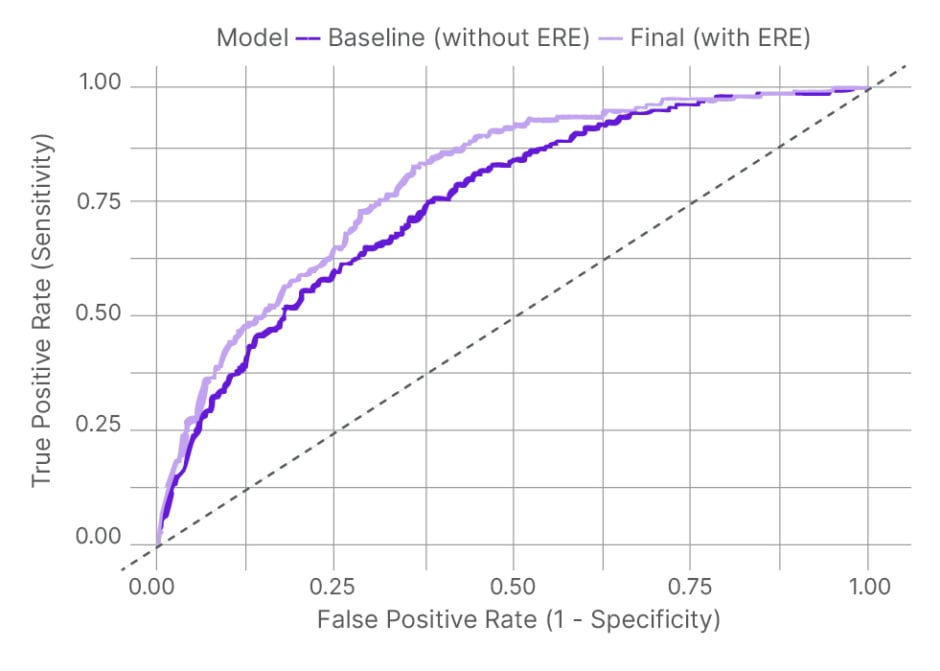
Figure 1: Area under the receiver operating characteristic curve comparison for baseline risk model versus ECG-based risk estimator model.
ERE: ECG-based risk estimator.
CONCLUSION
These findings suggest that a CNN-derived ECG risk score meaningfully improves short-term risk prediction in patients presenting with chest pain, offering additive value to existing triage models. The absence of events among patients with very low scores highlights its potential role in early rule-out strategies, while its independence from troponin indicates complementary clinical utility. Once validated prospectively, this tool could enable earlier and more precise decision-making in the emergency department, ultimately improving outcomes for patients with acute chest pain.