BACKGROUND
Cardiac biomarkers, such as troponins, are indicative of myocardial damage and can be detected in circulation. Not only are they frequently elevated following non-cardiac surgery, but clinically silent troponin elevations are associated with mortality and adverse cardiac events. As such, many patients are evaluated for myocardial injury after non-cardiac surgery, defined as one or more post-operative troponin measurements exceeding the 99th percentile upper reference limit of normal within 30 days of surgery.1 Ischaemic features, such as clinical symptoms or ischaemic electrocardiographic findings, are not required for the diagnosis of myocardial injury after non-cardiac surgery.1,2 However, patients with end-stage kidney disease (ESKD) present a unique challenge, as they often have elevated baseline troponin levels prior to surgery in the absence of myocardial ischaemia. As such, clinicians are not able to reliably interpret post-operative troponin measurements in patients with ESKD following non-cardiac surgery. Given that cardiovascular disease and its associated complications remain the leading cause of death in kidney transplant (KT) recipients with a functioning kidney allograft,3 it is imperative that the authors reflect on current pre-KT cardiac risk assessment practices. To date, no studies have examined the utility of baseline troponin levels in patients with ESKD as a tool to risk-stratify patients prior to KT. This study aims to evaluate the association between pre-transplant baseline troponin levels and the incidence of major adverse cardiac events (MACE) in the year following KT.4
METHODS
This is a retrospective cohort study at a tertiary care centre in Vancouver, Canada, consisting of 261 patients with ESKD and cardiovascular disease referred for assessment prior to KT between January 2013–January 2024. The primary endpoint was MACE in the year following KT, defined as all-cause death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularisation, heart failure hospitalisation, or cardiac arrest. Patients undergoing multiorgan transplantation or lacking baseline troponin measurements were excluded.
RESULTS
Of the 261 patients referred, 153 (59%) had a KT, 83 (32%) died pre-KT, and 25 (9%) were removed from the transplant programme (Figure 1). Among the 153 patients who received a transplant, 136 met the inclusion criteria. Of these, 24 patients (18%) experienced MACE within 1 year of KT. Elevated baseline troponin levels, defined as exceeding the 99th percentile upper reference limit of normal, were present in 93 patients (68%), while 43 (32%) had non-elevated troponin levels. Pre-KT demographics and comorbidities were comparable between the two groups. MACE in the year following KT was more prevalent in patients with elevated baseline troponin levels (22.6% versus 7.0%; p=0.026). Additionally, among those who developed MACE, baseline troponin levels were markedly greater (4.2 times versus 3.2 times the upper reference limit; p=0.048). Elevated troponin was associated with a nearly four-fold increased risk of MACE within 1 year of KT (odds ratio: 3.9; 95% CI: 1.1–13.8; p=0.036).
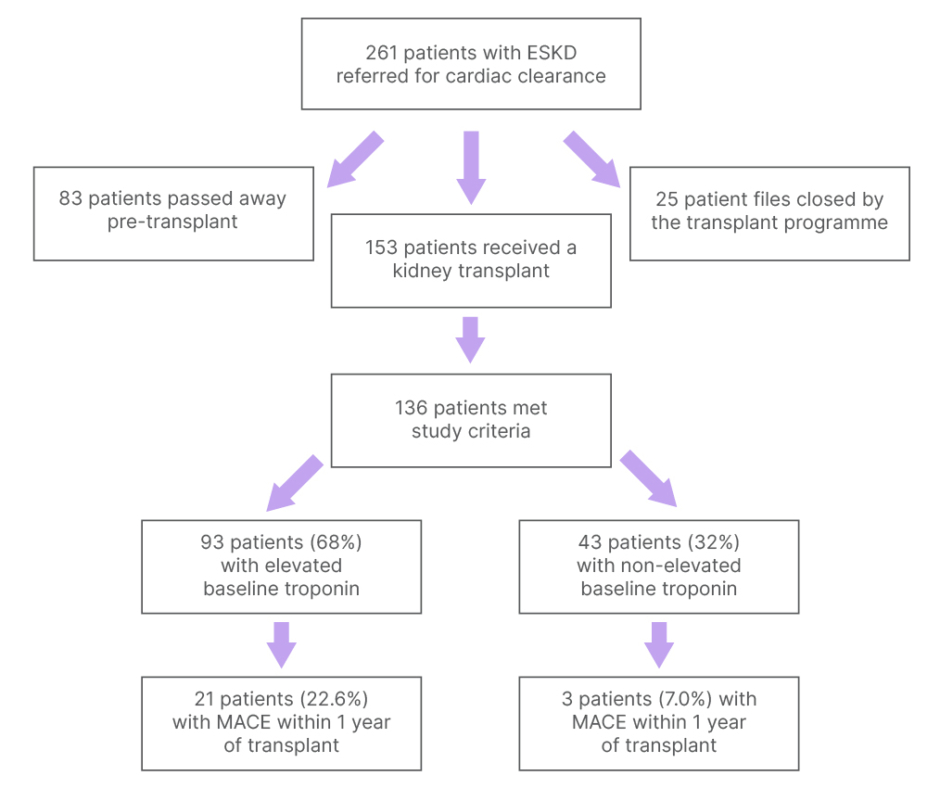
Figure 1: Flowchart of patients with end-stage kidney disease referred to the authors’ clinic during the study period.
KT recipients meeting study criteria are differentiated by elevated versus non-elevated baseline troponin.
ESKD: end-stage kidney disease; KT: kidney transplant; MACE: major adverse cardiac events.
CONCLUSION
Among patients with ESKD and cardiovascular disease, elevated baseline troponins were predictive of MACE in the year following KT. The authors’ study demonstrates the potential for baseline troponin measurements to enhance current, pre-KT cardiac risk stratification practices and act as an objective indicator of post-operative morbidity and mortality risk. However, further evaluation with long-term studies and larger cohorts remains necessary to validate the clinical utility of baseline troponin levels in risk stratifying patients with ESKD for adverse outcomes such as MACE following KT.