Abstract
Tetralogy of Fallot with pulmonary atresia and major aortopulmonary collateral arteries (MAPCA) represents the most severe variant of this congenital heart disease, with complete atresia of the pulmonary valve replacing pulmonary stenosis. Pregnancy in women with unrepaired lesions of this complexity is exceedingly rare and associated with major maternal and fetal risks. The authors report the case of an 18-year-old primigravida presenting at 31 weeks’ gestation with progressive dyspnoea, cyanosis, and peripheral oedema. Clinical evaluation and echocardiography revealed unrepaired tetralogy of Fallot with pulmonary atresia, a large ventricular septal defect, and MAPCAs. She was managed with diuretics, nitrates, antiplatelet therapy, and prophylactic heparin. Cardiac CT confirmed the diagnosis, demonstrating absent native pulmonary arteries and a MAPCA arising from the aortic root. Despite medical stabilisation and multidisciplinary care, she required Caesarean delivery at 35 weeks for fetal distress. A live but premature infant weighing 1.1 kg was delivered; however, neonatal demise occurred on Day 3. The mother remained stable post-partum and was discharged on medical management, though she declined surgical intervention. This case highlights the profound challenges of managing pregnancy in unrepaired tetralogy of Fallot with pulmonary atresia and MAPCAs, particularly in resource-limited settings. It underscores the need for early pre-pregnancy counselling, risk stratification, and coordinated multidisciplinary care in specialised centres to improve outcomes for both mother and child.
Key Points
1. Tetralogy of Fallot with pulmonary atresia and major aorto-pulmonary collateral arteries is an exceptionally rare condition, and its occurrence during pregnancy poses significant risks to both mother and fetus.2. In unrepaired tetralogy of Fallot, epidural or combined spinal-epidural analgesia is generally recommended, as it minimises abrupt reductions in systemic vascular resistance and helps prevent worsening of right-to-left shunting.
3. Early counselling and planning around contraception, pregnancy timing, and maternal/fetal risk are essential for women with congenital heart disease, to reduce complications and improve outcomes.
INTRODUCTION
Tetralogy of Fallot (TOF) is one of the most common forms of cyanotic congenital heart disease, characterised by right ventricular hypertrophy, an overriding aorta, a ventricular septal defect, and pulmonary stenosis. A less frequent anatomical variant occurs when pulmonary stenosis is replaced by pulmonary atresia. This form has an incidence of approximately 0.07 per 1,000 live births, accounting for up to 2.5% of all congenital heart diseases and around 20.0% of all TOF cases.1
In pulmonary atresia, the atretic pulmonary valve prevents blood flow through the right ventricular outflow tract, and pulmonary perfusion is therefore supplied via major aorto-pulmonary collateral arteries (MAPCA). The Congenital Heart Surgery Nomenclature and Database Project classifies TOF with pulmonary atresia into three subtypes according to pulmonary circulation: Type A, native pulmonary arteries present without MAPCAs; Type B, native pulmonary arteries present with MAPCAs; and Type C, MAPCAs only.1
The rarity of unrepaired TOF in pregnancy means that management strategies are not standardised and must be guided by case reports, expert opinion, and multidisciplinary collaboration. In resource-limited settings, the challenges are compounded by delayed diagnosis, limited access to advanced imaging, and restricted availability of surgical or interventional options.
Here, the authors report a rare case of unrepaired TOF with pulmonary atresia and MAPCA Type C in an adolescent primigravida. The patient experienced a premature pregnancy complicated by maternal cyanosis and neonatal demise. This case underscores the unique challenges of managing congenital heart disease in pregnancy, particularly in low-resource contexts, and adds to the limited literature on outcomes of unrepaired TOF with pulmonary atresia in young women.
CASE PRESENTATION
An 18-year-old primigravida presented at 31 weeks’ gestation (by dates) with a 2-week history of progressive shortness of breath (worse on minimal exertion; New York Heart Association [NYHA] Class III) associated with gradual, non-painful, bilateral lower-limb swelling. On examination, she was conscious, afebrile, and not pale, with central and peripheral cyanosis, Grade 3 digital clubbing, and bilateral pitting oedema of the lower limbs. Her initial observations were a blood pressure of 130/78 mmHg, a heart rate of 88 beats/min, a respiratory rate of 24 breaths/min (tachypnoeic), and an arterial O2 saturation of 86% on room air by pulse oximetry.
Cardiovascular examination revealed a regular, good-volume pulse synchronous with peripheral pulses. The jugular venous pressure was raised, at 8 cm H₂O. The apex beat was displaced, and a holosystolic murmur was audible at the left lower sternal border. A respiratory examination demonstrated diffuse bilateral basal fine crackles. An abdominal examination revealed a gravid uterus with a fundal height of 32 weeks and a fetal heart rate of 136 beats/min.
Initial laboratory investigations showed a haemoglobin of 15.0 g/dL, a haematocrit of 45.0%, and a platelet count of 197×10⁹ /L. Chest radiography demonstrated cardiomegaly (Figure 1).
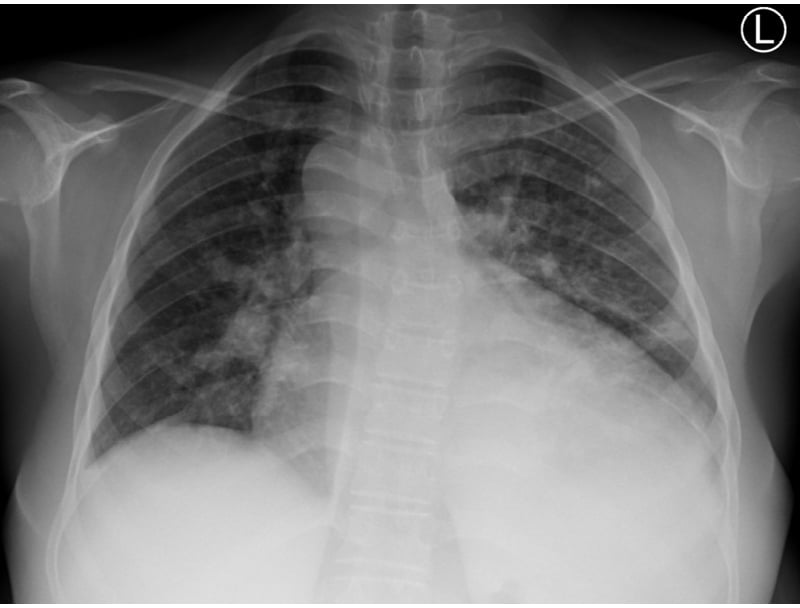
Figure 1: Chest radiograph.
Radiograph demonstrates a markedly enlarged cardiac silhouette consistent with moderate cardiomegaly. Increased pulmonary vascular markings (pulmonary plethora) are visible, suggestive of a left-to-right shunt.
Electrocardiography showed features of right heart strain. Transthoracic echocardiography revealed situs solitus with laevocardia, dilated right atrium, hypertrophied right ventricle, a large inlet ventricular septal defect (20 mm) with bidirectional shunt, an overriding aorta (>85% of the conotruncal artery), and pulmonary atresia. A right aortic arch with collateral arteries arising from the aorta was noted. Left ventricular function and valvular anatomy were preserved, with minimal pericardial effusion (Figure 2).
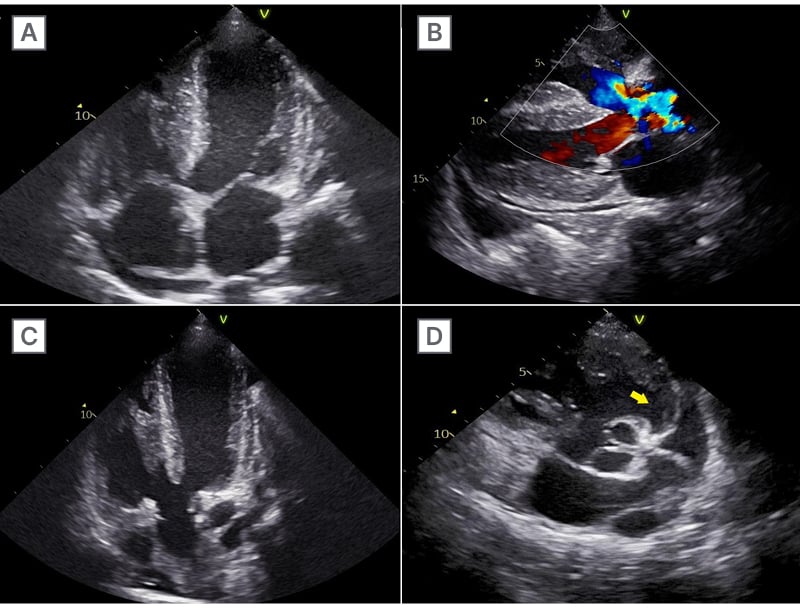
Figure 2: Transthoracic echocardiography.
Echocardiography shows: A, B) situs solitus with levocardia, a dilated right atrium, a hypertrophied right ventricle, and a large inlet ventricular septal defect (20 mm) with bidirectional shunting; C) an overriding aorta involving >85% of the conotruncal artery; and D) pulmonary valve atresia (yellow arrow).
She was started on intravenous furosemide 40 mg twice daily, oral isosorbide dinitrate 5 mg twice daily, oral acetylsalicylic acid 75 mg once daily, and subcutaneous unfractionated heparin 7,500 U twice daily. Under lead protection, a cardiac CT scan was performed, which confirmed TOF with a large ventricular septal defect. The pulmonary artery was not visualised, consistent with pulmonary atresia, and a MAPCA was identified arising from the sinotubular junction of the aortic root, providing alternative pulmonary blood supply (Figure 3).
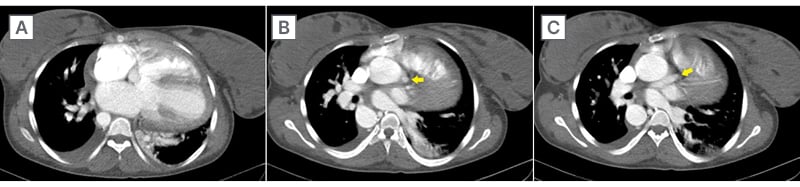
Figure 3: Cardiac CT.
The CT demonstrates: A) an overriding aorta with a large ventricular septal defect and absent pulmonary artery consistent with pulmonary atresia; and B, C) a MAPCA Type C arising from the sinotubular junction of the aortic root and supplying the pulmonary arteries (yellow arrow). The right atrium, left atrium, and left ventricle appear dilated. MAPCA: major aortopulmonary collateral artery.
The patient’s symptoms improved over the following days. A multidisciplinary team counselled her regarding maternal and fetal risks, but she and her family opted to continue with the pregnancy. She received intramuscular dexamethasone 6 mg twice daily for 48 hours to promote fetal lung maturation and was monitored closely. Follow-up laboratory tests revealed a haemoglobin of 16.5 g/dL, a haematocrit of 50.8%, and a platelet count of 171×10⁹ /L.
At 35 weeks’ gestation, due to fetal distress, a Caesarean section was performed under general anaesthesia. A live female infant weighing 1.1 kg was delivered, with Appearance, Pulse, Grimace, Activity, and Respiration (Apgar) scores of 6 and 8 at 1 and 10 minutes, respectively. The neonate was admitted to the premature unit but, unfortunately, died after 3 days. The mother remained clinically stable post-partum, and was monitored closely for 1 week without further deterioration. Counselling regarding her condition was provided prior to discharge.
At follow-up visits, additional counselling addressed future pregnancies, contraceptive options, and surgical intervention. However, the patient and her family were reluctant to proceed with surgery and chose to continue with medical management.
DISCUSSION
Unrepaired TOF that persists into adulthood is a rare occurrence, and even rarer in pregnancy. Adult patients with TOF usually present according to the severity of the underlying anatomical defects, with common manifestations including heart failure, palpitations, arrhythmias, syncope, and right ventricular dysfunction.2 Fewer than 1% of unrepaired TOF cases survive into adulthood, as early surgical repair is considered the definitive management. However, in patients with pulmonary atresia, survival may be facilitated by the development of pulmonary collaterals and the absence of additional congenital malformations.3
The burden of congenital heart disease is lower in Africa compared with high-income regions. The prevalence has been estimated as two per 1,000 live births in Africa, compared with eight and nine per 1,000 in Europe and Asia, respectively.3 The incidence of TOF with pulmonary atresia is approximately 0.7 per 100,000 live births, representing around one-fifth of all TOF cases.4 In Tanzania, the prevalence of TOF with pulmonary atresia has been reported as 1.2% of congenital heart disease cases.5 Unrepaired TOF is predominantly reported in developing countries, with a 10-year survival of less than 8%. In contrast, surgical repair increases survival to approximately 80%.6 Factors influencing outcomes include abnormalities of the pulmonary arteries, elevated right ventricular systolic pressure, arterial O2 saturation below 85%, a haemoglobin of >20 g/dL, and haematocrit >65%.2
Disease-specific maternal cardiovascular risk assessment is best undertaken using the modified WHO classification, which remains the most widely adopted system. However, its utility is limited in low-resource settings.7 In this case, the patient was categorised as modified WHO Class III, reflecting unrepaired cyanotic congenital heart disease.
Pregnancy induces profound physiological adaptations, particularly within the cardiopulmonary system. These include an increase in maternal plasma volume by approximately 50%, erythrocyte mass by approximately 40%, and total blood volume by approximately 45% by 32 weeks’ gestation.8-10 There is also an increase in cardiac output of around 45% by 24 weeks, mediated by both stroke volume and heart rate, and facilitated by reduced afterload, though end-diastolic pressures, ejection fraction, and central and pulmonary pressures remain largely unchanged.9,10 Systemic vascular resistance decreases by approximately 30% by mid-pregnancy before rising again near term.10 Maternal serum albumin falls by about 18% at 24 weeks, lowering colloid oncotic pressure and predisposing to pulmonary oedema after crystalloid infusion.10 In normal pregnancies, mean pulmonary wedge pressure is around 7.5 mmHg, pulmonary congestion develops above 18 mmHg, and oedema typically develops after ≥28 mmHg.11,12 Respiratory adaptations include increases in tidal volume and minute ventilation by approximately 40%, and O2 consumption by approximately 15%, producing around a 25% reduction in maternal arterial partial pressure of CO2.13,14 Maternal arterial O2 tension rises slightly due to hyperventilation and enhanced cardiac output; however, O2 saturation should remain >95% in both rest and exertion.15
In women with TOF, these physiological changes exacerbate right-to-left shunting, further lowering systemic O2 saturation and worsening hypoxaemia.10,16 Reduced cardiac reserve predisposes women to decompensation.10,17 Non-cardiac dyspnoea in patients with cyanosis may also result from the altered gas exchange and pH changes associated with the augmented right-to-left shunt during pregnancy and exercise.15,18 Maternal mortality is particularly high in those with co-existing pulmonary hypertension, even when they are in functional Class II before pregnancy. In such cases, acute increases in pulmonary vascular resistance due to haemorrhage, anaesthesia, or haemodynamic shifts can precipitate sudden death.19,20
Cardiac catheterisation remains the reference standard for defining the pulmonary arterial tree and delineating MAPCA anatomy in TOF with pulmonary atresia. It enables selective angiography of individual collaterals, assessment of segmental pulmonary perfusion, and quantification of shunt and pulmonary vascular resistance. These data are crucial in determining surgical options, such as staged or single-stage unifocalisation and suitability for a right ventricular–pulmonary artery conduit. Cardiac catheterisation can also identify collaterals supplying non-functional lung segments.1 In specialist centres, catheter-based interventions may be employed to support surgical management, including coil embolisation of competing collaterals, occlusion of non-contributory high-flow MAPCAs, and balloon angioplasty or stenting of stenotic collaterals to optimise downstream perfusion.1,2
During pregnancy, diagnostic catheterisation is generally avoided unless results are expected to alter immediate management. When required, measures such as abdominal shielding, reduced fluoroscopy frame rates, collimation, and minimised exposure times are essential, with the second trimester preferred where feasible.2,6 In the authors’ patient, detailed anatomical definition was achieved using cardiac CT imaging. Invasive haemodynamic assessment was therefore deferred, in keeping with the principle that catheterisation should only be undertaken if results would significantly change management.1,6
Vaginal delivery is associated with an acute rise in cardiac output (up to 80%) due to caval decompression and the autotransfusion of blood from the contracting uterus into the maternal circulation.10 This abrupt increase in venous return elevates pulmonary blood volume and flow but usually returns to pre-labour levels within an hour post-partum. By comparison, Caesarean delivery is accompanied by greater blood loss, which partially blunts this increase in venous return.13 Cardiac output peaks around 15 minutes post-partum, with maximum increments of approximately 37% under epidural anaesthesia and approximately 28% under general anaesthesia.10,20 Stroke volume and cardiac output may remain modestly elevated for up to a year post-partum, and haemodynamic responses are augmented in subsequent pregnancies. Thus, pregnancy induces a permanent remodelling of the maternal cardiovascular system.10,13
Pregnancy in unrepaired TOF is associated with serious risks, including maternal morbidity up to 65%, maternal mortality up to 15%, and adverse perinatal outcomes.21,22 Management is therefore vital to minimise complications. Haemodynamic changes in pregnancy may exacerbate right-to-left shunting, precipitating hypoxaemia, cyanosis, and increased risks of both maternal and fetal death.23 Chronic hypoxia also drives secondary polycythaemia, which contributes to hyperviscosity and thrombosis.24
Cyanotic congenital heart disease confers a complex coagulation profile. Secondary erythrocytosis and haemoconcentration elevate thrombotic risk, yet iron deficiency, thrombocytopenia, platelet dysfunction, and reduced clotting factor levels predispose to haemorrhage. Pregnancy adds another layer of hypercoagulability and plasma volume shifts, further amplifying risks of both thrombosis and bleeding during the peripartum period.2,6,7
Management strategies should therefore include: a) careful hydration with avoidance of unnecessary venesection; b) active investigation and correction of iron deficiency to reduce hyperviscosity without resorting to routine phlebotomy; c) individualised decisions on antithrombotic therapy, typically low-dose aspirin and/or prophylactic heparin, based on cyanosis, immobility, and obstetric risk; and d) multidisciplinary peripartum planning to avoid haemodynamic instability.2,7 Therapeutic phlebotomy is reserved for patients with symptomatic hyperviscosity and very high haematocrit, and should be combined with volume replacement to avoid circulatory collapse.6,7 Post-partum, early mobilisation and extended thromboprophylaxis may be appropriate, provided bleeding risk is controlled.2,7
Maternal complications in TOF include right ventricular dilatation and failure, worsening cyanosis, dyspnoea, arrhythmias, thromboembolism, aortic root dilatation, endocarditis, and maternal death. Obstetric complications include miscarriage and postpartum haemorrhage,25 while fetal complications include abortion (approximately 20%), intrauterine growth restriction (approximately 50%), prematurity (approximately 50%), and fetal loss (approximately 15%).2,26 In the authors’ case, the neonate was premature with growth restriction and died within 3 days. Maternal cyanosis, ventricular dysfunction, and polycythaemia were the main contributory factors.26
The optimal mode of delivery is determined by balancing maternal and fetal risks. Vaginal delivery is usually preferred due to lower risks of haemorrhage, thromboembolism, infection, pulmonary congestion, and maternal death. Caesarean delivery is reserved for obstetric indications, and regional anaesthesia is generally favoured over general anaesthesia owing to better haemodynamic stability, absence of respiratory depression, and readiness for urgent operative delivery.16 Epidural or combined spinal-epidural anaesthesia reduces abrupt decreases in systemic vascular resistance and may mitigate worsening of right-to-left shunting. In contrast, general anaesthesia with intermittent positive-pressure ventilation can increase pulmonary vascular resistance and exacerbate shunting.16 If general anaesthesia is required, meticulous attention to maintaining systemic and pulmonary vascular resistance balance is crucial.2
After delivery, the prevention of haemorrhage is essential. Oxytocin remains first-line, but must be administered cautiously, as it can provoke hypotension and reflex tachycardia, thereby worsening shunting.16 Methylergometrine may be preferable for uterine tone, as it preserves systemic vascular resistance.23 Careful postpartum monitoring is imperative, especially in the first 48 hours, when cardiovascular adaptations are most pronounced.2,16
Published literature on pregnancy in TOF, spanning repaired and unrepaired anatomies, consistently demonstrates that maternal and fetal outcomes are primarily determined by cyanosis, right-sided pressures, and the presence of residual shunting. Reports already cited in this manuscript2,4,21,23,25,26 highlight the high rates of prematurity, growth restriction, and fetal loss in unrepaired TOF, with comparatively improved outcomes following surgical repair or when maternal O2 saturation is ≥90%. Caesarean delivery is typically reserved for obstetric reasons, and regional anaesthesia is preferred when feasible, reflecting current guideline-based practice.2,7
The authors’ case echoes these themes: delayed presentation, the constraints of a resource-limited setting, and neonatal vulnerability. It contributes new insights by a) demonstrating detailed multimodality imaging consistent with TOF with pulmonary atresia and MAPCAs; b) illustrating the rationale for deferring catheterisation until post-partum; and c) outlining a pragmatic haemostatic strategy tailored to a low-resource environment. Collectively, these features extend the available evidence from sub-Saharan Africa and underscore the importance of pre-pregnancy counselling, multidisciplinary care, and planned delivery in centres equipped with adult congenital cardiology and high-risk obstetric expertise.2,4,21,23,25,26
CONCLUSION
TOF with pulmonary atresia and MAPCAs is exceptionally rare, and its occurrence during pregnancy poses major challenges for both maternal and neonatal outcomes. Effective management requires early recognition, comprehensive counselling, and coordinated multidisciplinary care. Where possible, delivery should be planned in centres with specialised cardiac and neonatal expertise to optimise survival and reduce the risks of maternal and perinatal mortality.