BACKGROUND AND AIMS
Onychomycosis is a prevalent and difficult-to-treat nail infection. Although both oral and topical antifungals are available, systemic agents such as terbinafine and itraconazole are often limited by drug interactions and side-effects.1,2 Consequently, there is a clinical need for safer and effective topical therapies.3-5 This study evaluated the efficacy and tolerability of a novel topical antifungal formulation in patients with distal lateral subungual onychomycosis.6
MATERIALS AND METHODS
This single-arm, open-label clinical trial enrolled 50 adult subjects with clinically diagnosed distal lateral subungual onychomycosis (≤30% nail involvement, at least one big toe). The tested topical solution, containing urea, lactic acid, ethoxydiglycol, decylene glycol, and polyquaternium-7, was applied twice daily for 6 months. Outcome measures included blinded investigator global assessment, mycological evaluation (potassium hydroxide microscopy and culture), and patient self-assessment of efficacy and usability. Nail growth (mm) was periodically monitored. Complete cure was defined as negative mycology and resolution of clinical signs. Safety was assessed through adverse event reporting.
RESULTS
Forty patients completed the study. At 3 months, 80% of participants achieved mycological negativity (direct microscopy + culture). At 6 months, the complete cure rate was 50.0% and an additional 42.5% showed significant clinical improvement (Figure 1). Mean healthy nail growth was 1.40 mm at Week 4, and after 6 months, the mean growth reached 3.55 mm. A blinded investigator assessed improvement from standardised photographs. Statistically significant changes were observed over time (p<0.001; Friedman test). At Week 4, eight patients (20.0%) showed excellent improvement and 29 (72.5%) moderate improvement. At Week 12 (Month 3), 13 (32.5%) showed excellent improvement and 24 (60.0%) moderate improvement. At Month 6, 19 patients (47.5%) showed excellent and 17 (42.5%) moderate improvement (p<0.001 for all timepoints versus baseline; Wilcoxon test with Bonferroni correction).
Patient satisfaction at 6 months was 95%, and investigators rated the treatment positively in 87.5% of cases. Four patients developed mild periungual erythema due to improper application; all cases resolved without discontinuation.
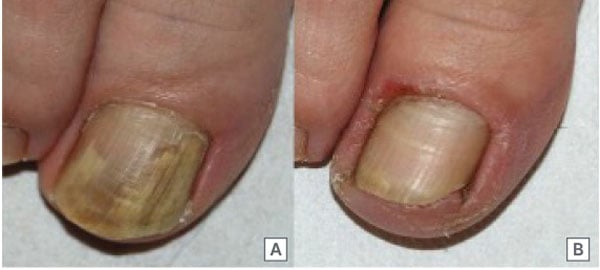
Figure 1: Excellent improvement (complete cure) of Trichophyton rubrum distal lateral subungual onychomycosis (A) from baseline and (B) after 3 months of treatment (Week 12).
CONCLUSION
Onychomycosis remains a challenging condition to manage with topical therapies, due to the nail plate’s poor permeability and slow growth rate, both of which reduce the effectiveness of antifungal agents and require prolonged treatment durations to achieve clinical resolution. This topical antifungal solution demonstrated clinically meaningful improvements in nail appearance and fungal resolution already after 12 weeks, with further benefit at 6 months. The formulation forms a protective film on the nail surface, which acts as a barrier against environmental pathogens.5,7 Moreover, polyquaternium-7, decylene glycol, and urea contribute to nail hydration and barrier integrity. Lactic acid helps to maintain an acidic pH, unfavourable for fungal growth.4,7,8 Also, a combination ointment including urea exhibited better antifungal properties due to urea helping the penetration of active pharmacological agents.4 The formulation was safe, well tolerated, and easy to use. Its non-pharmacological mechanism of action may allow for concomitant use with systemic antifungals when indicated.







