INTRODUCTION
Skin photosensitivity is defined as the cutaneous response to UV radiation (UVR) and/or visible light (VL). The Fitzpatrick Skin Phototype Classification (FSPC) has been widely adopted as a method for assessing an individual’s baseline susceptibility to sunburn and tanning.1 Photosensitivity is deemed pathological when the skin’s reaction to UVR/VL deviates significantly, either qualitatively or quantitatively, from that of the general population. Clinical manifestations, including the development of rashes or exaggerated sunburn-like reactions following minimal UVR/VL exposure, indicate an underlying abnormal photosensitivity disorder.2 These photosensitivity disorders are provoked or aggravated by sunlight or artificial light sources and exhibit wide variability in both prevalence and clinical severity. Polymorphic light eruption (PLE) is among the most common, with a pooled estimated prevalence of 10.00% among the general population (ranging from 0.65% in China to 21.40% in Ireland),3 whereas solar urticaria is rare, with a point prevalence of 3.1 per 100,000 in the Tayside region of Scotland, UK.4
Management of these conditions often requires substantial lifestyle adaptations, including strict photoprotection and reduced outdoor activity. Beyond the physical manifestations, the psychosocial impact is considerable. A systematic review by Rutter et al.5 found that 31–39% of patients reported a very large impairment in quality of life (Dermatology Life Quality Index [DLQI] >10), highlighting the broader burden imposed by photodermatoses and underscoring the importance of accurate diagnosis of abnormal cutaneous photosensitivity through thorough clinical assessment and appropriate investigations. Given the heterogeneity of the clinical presentations, patients may initially present to a range of medical specialists. This feature aims to review the clinical features, diagnostic approaches, and general management strategies for these conditions.
Although photodermatoses are traditionally classified by pathogenesis, for the purposes of this feature, the authors propose an alternative classification based on the timing of clinical presentation, which may be more practical for the general physician. Photodermatoses can be broadly classified into three categories: those with immediate reactions (seconds–minutes) following sun exposure, those with intermediate responses (minutes–hours), and those with delayed responses (hours–days), often associated with more chronic manifestations over several days–weeks. Some causes of photosensitivity can also lead to delayed problems, including skin cancers. Regardless of the classification system used, a thorough clinical assessment is essential to identify a history of photosensitivity, characterise the nature of the reaction, and guide diagnostic evaluation.
Classification of the photodermatoses based on pathogenesis:6
- Immunological
- Polymorphic light eruption
- Juvenile spring eruption
- Solar urticaria
- Chronic actinic dermatitis
- Actinic prurigo
- Hydroa vaccinforme
- Drugs and chemicals
- Endogenous: porphyrias
- Exogenous: systemic and topical
- Photogenodermatoses
- Xeroderma pigmentosum
- Bloom syndrome
- Rothmund–Thompson syndrome and others
- Photoaggravated
- Atopic dermatitis
- Seborrhoeic dermatitis
- Connective tissue diseases, e.g., lupus, dermatomyositis
- Rosacea and others
IMMEDIATE-ONSET PHOTODERMATOSES (TRIGGERED WITHIN SECONDS–MINUTES OF EXPOSURE)
Solar urticaria (Figure 1) is an immediate-type hypersensitivity reaction that can occur at any age and is characterised by immediate onset (within seconds–minutes of exposure) of itching, erythema, and whealing on sun-exposed sites, which resolve in ≤1 hour in 64.1% and persist up to 24 hours in 33.5%. As with other inducible urticarias, a specific underlying cause is not identified.7

Figure 1: Solar urticaria.
Patient affected on exposed feet, exhibiting sparing on legs covered by trousers.
Erythropoietic protoporphyria (EPP) is an acute inherited disorder and should be suspected in patients presenting with skin pain after a few minutes of sun exposure. A typical first manifestation of EPP is a baby crying when put outdoors in daylight or when exposed to light coming through the window glass.8
A severe sunburn reaction occurring after trivial quantities of sunlight and persisting beyond the expected duration of a ‘normal sunburn’ may indicate xeroderma pigmentosum (XP), a rare autosomal recessive condition. Patients with XP may have associated features such as persistent freckles (lentigines) of sunlight-exposed skin, as well as an increased susceptibility to cutaneous malignancy from as young as 5 years of age.9
A careful drug history is of utmost importance in any patient presenting with signs or symptoms of photosensitivity. Immediate prickling, burning, or erythema is particularly associated with drugs such as amiodarone and chlorpromazine, whereas an exaggerated sunburn-like reaction can be associated with fluoroquinolone or tetracycline antibiotics, amiodarone, or thiazide diuretics.10
INTERMEDIATE-ONSET PHOTODERMATOSES (TRIGGERED BY MINUTES–HOURS OF EXPOSURE)
PLE (Figure 2), the most common photodermatosis, usually develops in spring or summer and is typically triggered by 30 minutes–several hours of sun exposure, often occurring later that day or the next day and resolving without scarring within days, if further sun exposure is avoided. Several morphological subtypes have been described, although a pruritic, erythematous, papular, and sometimes vesicular rash on non-perennially exposed sites such as the chest and arms is the most common presentation.11 Many patients experience symptomatic improvement by late summer, likely due to a gradual ‘hardening’ or increased phototolerance of the skin. Some individuals may only exhibit symptoms during periods of intense sun exposure, such as sunny holidays, whereas those with more severe forms may experience symptoms year-round. The diagnosis of PLE is primarily clinical; however, phototesting and/or histopathological evaluation may be necessary in atypical or unclear cases.12
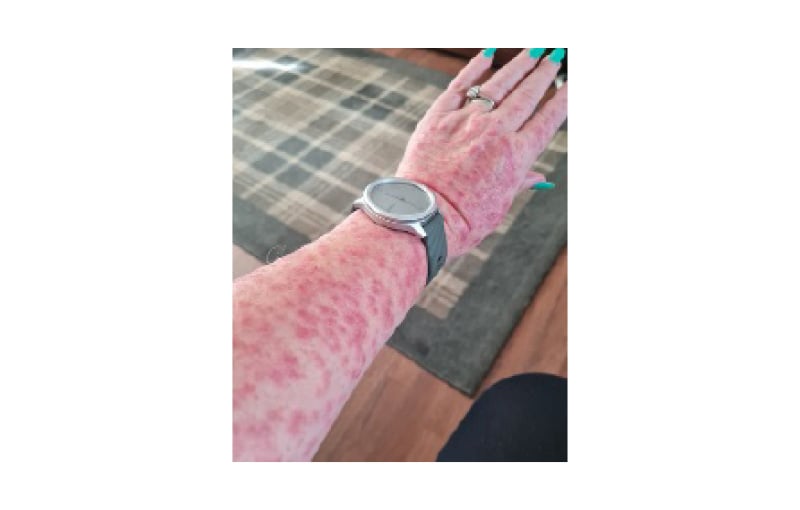
Figure 2: Polymorphic light eruption.
Erythematous papular eruption on forearms.
DELAYED-ONSET PHOTODERMATOSES (TRIGGERED BY HOURS–DAYS OF EXPOSURE)
Drug-induced photosensitivity can present not only with acute reactions but also with a range of delayed manifestations. These include increased skin fragility and blistering due to trauma in pseudoporphyria (commonly linked to drugs such as naproxen and furosemide), and telangiectasia in photo-exposed areas, particularly with calcium channel blockers.10
Chronic actinic dermatitis (Figure 3) should be considered in patients presenting with eczematous eruptions in a photo-distributed pattern, or in those with pre-existing dermatitis (sometimes atopic) who develop a change in distribution or seasonal pattern of flares. As patients may not associate their symptoms with sun exposure, a high index of clinical suspicion is warranted.6
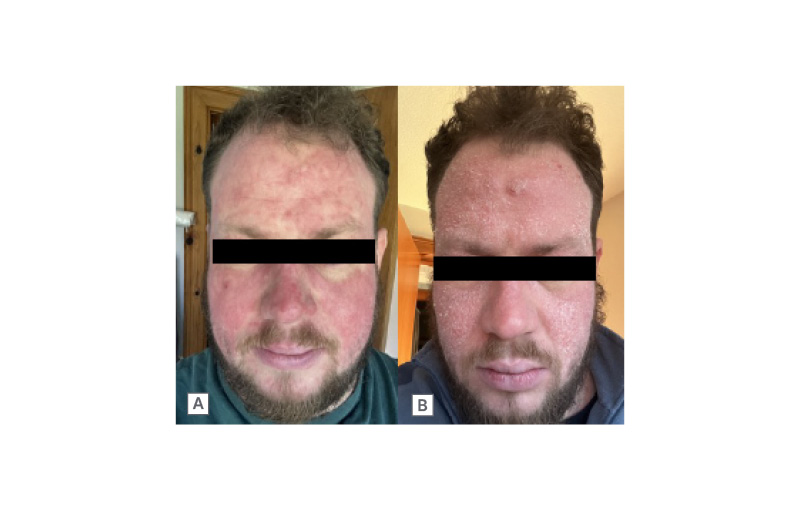
Figure 3: Chronic actinic dermatitis.
A photo-exposed site dermatitis showing acute (A) and chronic (B) dermatitic reaction. Note the relative sparing in photoprotected areas.
The presence of scarring in photo-exposed areas should prompt consideration of conditions such as actinic prurigo or hydroa vacciniforme. Actinic prurigo is an immunological photodermatosis, most prevalent among individuals of American Indian descent, and typically presents in childhood. In the UK, it is closely associated with human leukocyte antigen (HLA) DR4 and the subtype DRB1*0407.13 It is usually perennial and begins with sunlight-induced pruritic, patchy, and oedematous erythema accompanied by papules and occasional vesicles. Over time, excoriated prurigo nodules and papules develop, often healing with post-inflammatory changes including pitted or linear facial scars.14
In contrast, hydroa vacciniforme presents with itchy, tender papules, oedema, and haemorrhagic crusting that resolve with characteristic varioliform scarring.15 This condition is extremely rare in the UK, but needs to be distinguished from the potentially life-threatening Epstein–Barr virus-driven hydroa vacciniforme-like presentation of a lymphoproliferative disorder, which is more often reported in Asia.16
Porphyria cutanea tarda (PCT) is the most common form of cutaneous porphyria in Europe and is usually acquired. It is frequently associated with excessive alcohol consumption, chronic hepatitis C infection, autoimmune hepatitis, and haemochromatosis. Clinically, PCT typically presents with skin fragility, blistering, and scarring with milia on sun-exposed areas, particularly the dorsal hands. Early recognition is critical, as management focuses on treating the underlying hepatic pathology.17
PHOTOAGGRAVATED PHOTODERMATOSES
Photoaggravated photodermatoses constitute another relatively large group of conditions, which are exacerbated but not caused by light. Connective tissue diseases such as lupus and dermatomyositis have clinical features of photodistributed rashes, and dermatoses such as atopic dermatitis, seborrhoeic dermatitis, and allergic contact dermatitis may also be aggravated by light.18-20
INVESTIGATIONS
Patients suspected of having a photosensitivity disorder should be referred to a specialist photodiagnostic unit for comprehensive evaluation. The primary objectives of the investigation are to characterise the nature of the photosensitivity and establish a definitive diagnosis. The gold-standard diagnostic tool is monochromator phototesting, which uses a xenon-arc light source filtered to deliver narrow wavebands across the solar spectrum (Figure 4). This allows assessment of erythemal responses at specific wavelengths, which can then be compared with reference values from the normal population.
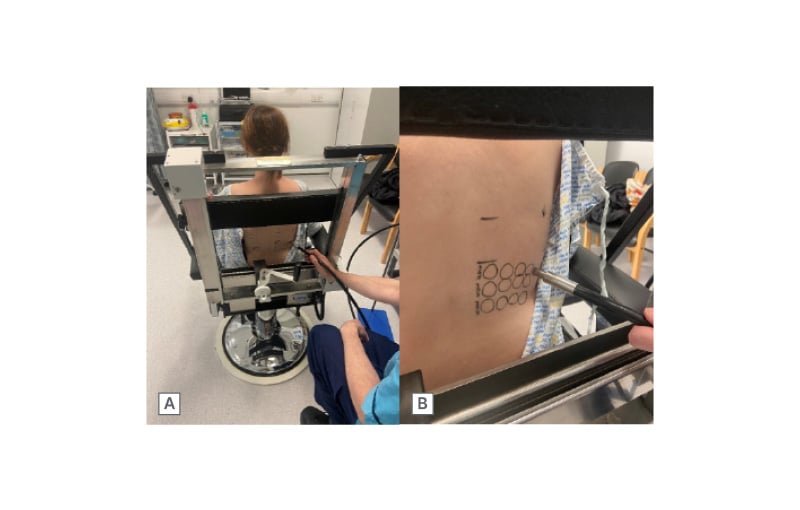
Figure 4: Monochromator phototesting.
A) Phototesting with the patient sitting comfortably. The fibre-optic light guide is connected to a filtered xenon arc source. B) Irradiation undertaken at test sites on clinically normal appearing back skin and delivered via fibre-optic light guide.
If monochromator phototesting is unavailable, determination of the minimal erythema dose (MED) can be attained using broadband UVB, UVA, and VL sources. MED, the lowest dose of radiation required to produce just perceptible erythema, is typically assessed 24 hours post-irradiation. MED serves as the primary endpoint in most photosensitivity disorders (Figure 5). In contrast, for conditions such as solar urticaria, the minimal urticaria dose, which is the smallest dose required to provoke detectable urticaria, serves as the principal diagnostic threshold. In some metabolic conditions, such as cutaneous porphyrias, abnormal photosensitivity is sometimes only observed at around 7 hours.
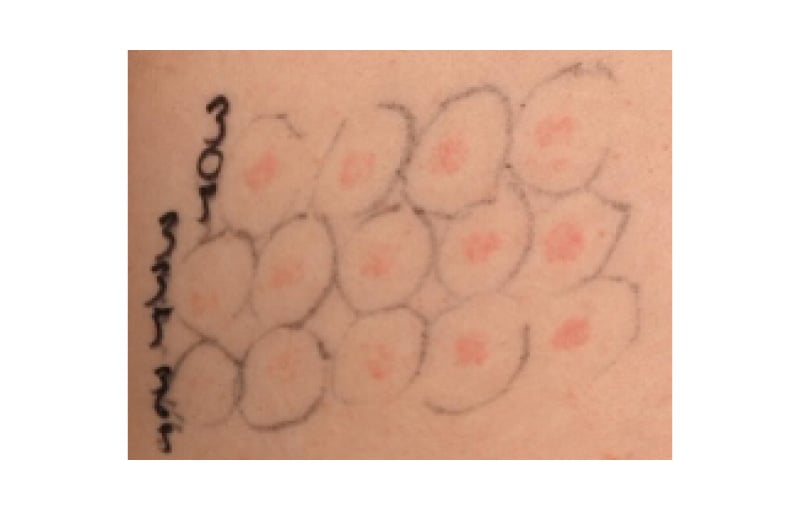
Figure 5: Positive monochromator testing in a patient with chronic actinic dermatitis.
Positive monochromator testing result in a patient with chronic actinic dermatitis showing abnormal UV sensitivity MED at 305±5 nm <1.5 (lowest normal 33) mJ/cm2; at 335±27 nm 180 (lowest normal 3,900) mJ/cm2 and at 365±27 nm 1,500 (lowest normal 18,000) mJ/cm2.
MED: minimal erythema dose.
Additional investigations may include UVA provocation testing and photopatch testing when photoallergic contact dermatitis (e.g., to sunscreen chemicals or topically applied non-steroidal anti-inflammatories) is suspected. Furthermore, MED testing (Figure 6) plays a critical role in phototherapy safety screening, identifying abnormal photosensitivity reactions and guiding the selection of accurate starting doses for treatment. Screening for connective-tissue disease, porphyrias, and rarer disorders (including HLA testing) may be indicated depending on the individual clinical presentation.21
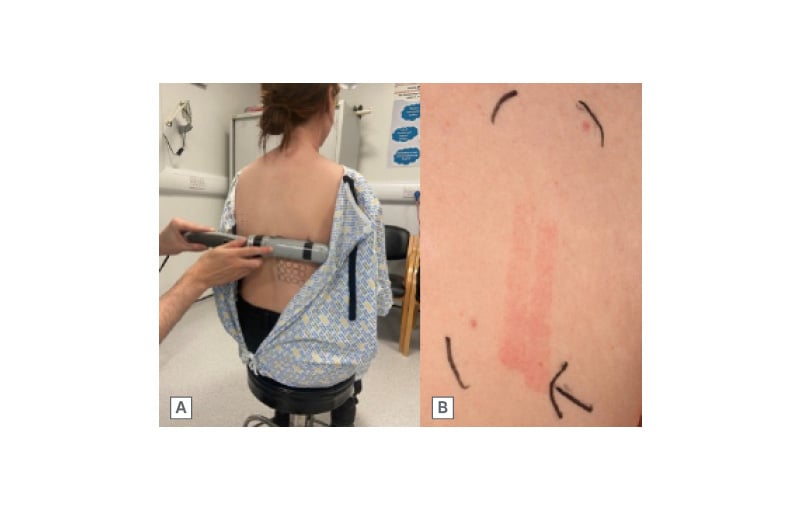
Figure 6: UVB minimal erythema dose testing.
A) Narrowband UVB MED testing using a handheld device with 10 fixed incremental doses of narrowband UVB. B) UVB MED testing result showing erythema at all 10 doses, indicating abnormal photosensitivity with a MED of <0.076 (lowest normal 0.079) J/cm2. The highest dose delivered was 0.4 J/cm2, indicated by the black arrow.
MED: minimal erythema dose.
GENERAL MANAGEMENT
General management principles for all photodermatoses begin with strict adherence to photoprotection, including broad-spectrum sunscreens, protective clothing, UV-filtering eyewear, and behavioural modifications to minimise sun exposure, such as seeking out shade. Patient education plays a crucial role in ensuring consistent and effective implementation of these measures.
Photohardening, a gradual increase in controlled sunlight exposure, may improve tolerance, particularly in PLE.22 Light-based therapies, such as narrowband UVB or psoralen-UVA phototherapy, may also be employed under specialist advice to induce tolerance in chronic photodermatoses and serve as both prophylactic and therapeutic interventions.
For more severe or refractory cases, immunosuppressive agents such as methotrexate, azathioprine, or ciclosporin may be considered, especially in conditions like chronic actinic dermatitis.23 Emerging therapies include afamelanotide, a synthetic α-melanocyte-stimulating hormone analogue, which has shown efficacy in improving sunlight tolerance and quality of life in EPP.24 In PCT, effective treatment of the underlying cause, such as direct-acting antiviral therapy for hepatitis C, has been transformative.25
Biologic therapies are also being explored, with omalizumab, an anti-IgE monoclonal antibody, demonstrating benefit in selected cases of solar urticaria refractory to conventional treatments.26 These advances highlight the importance of an individualised, multidisciplinary approach that adapts to disease severity, comorbidities, and evolving therapeutic options.
General management principles:
- Photoprotection
- Environmental factors
- Behavioural change, e.g., seeking shade
- Clothing and hat
- Broad-spectrum high protection factor sunscreens
- Window film
- Natural photohardening
- Vitamin D supplementation
- Topical treatments
- Emollients
- Topical corticosteroids
- Topical calcineurin inhibitors
- Light-based therapies
- Narrowband UVB
- Psoralen-UVA
- UVA1
- Systemic therapies
- Immunosuppressive agents, e.g., methotrexate, ciclosporin
- Immunomodulator agents, e.g., omalizumab, dupilumab
CONCLUSION
Photosensitivity disorders encompass a diverse group of conditions that can significantly impair quality of life and often lack definitive cures. Management remains holistic, aiming to minimise sun exposure, optimise skin protection, and address comorbidities and psychosocial impact. Despite their potentially debilitating impact, photosensitivity disorders remain underserved, with significant disparities in access to diagnostic and therapeutic services. A key research priority is the Global Assessment of Photodiagnostic Services (GAPS) project, designed to evaluate worldwide availability and quality of photodiagnostic services and guide targeted strategies to improve care.
Additionally, resources to support patients, ranging from specialist input to education and support groups, vary considerably, influencing both outcomes and patient experience. Emerging data, including recent updates on life expectancy in patients with XP,27 have provided critical insights into the long-term prognosis of specific photosensitivity disorders, emphasising the importance of early diagnosis, rigorous photoprotection, and coordinated care.
There remains an urgent need for greater awareness, improved access to specialist services, and continued research to refine diagnostic tools, optimise interventions, and ultimately enhance the lives of individuals affected by these conditions.







