Meeting Summary
Atopic dermatitis (AD) is a chronic, inflammatory skin condition, with approximately 30 million people in the USA estimated to have this disorder in 2024. The prevalence of AD and the tendency to develop severe disease are greater in patients with skin of color (SOC) than in White patients; however, patients with SOC are under-represented in clinical trials, and specific recommendations for these patients in the guidelines are limited. This article summarizes a poster, ‘Evaluation of natural moisturizing factors in diverse ethnically patients with atopic dermatitis following prebiotic skincare regimen’, presented as part of the Skin of Color Update Conference in New York, USA, held from October 3rd–5th, 2025. An exploratory study was conducted to investigate the properties of the skin barrier in 20 patients aged 3–80 years with AD from diverse racial/ethnic populations who were following a 10-week prebiotic skincare regimen comprising a cleanser and moisturizer. The results of the study showed that a prebiotic skincare regimen improves AD symptoms in patients from diverse racial/ethnic populations by restoring skin barrier integrity. Improvements were reported in disease severity, skin hydration, skin pH, stratum corneum isotropy total score, and levels of natural moisturizing factor (NMF). Notably, baseline NMF levels were lower in patients with SOC compared with White counterparts, which may at least in part explain the differences in AD prevalence and disease severity between these populations. Raising awareness of the variation in NMF levels in diverse racial/ethnic populations, as well as the potential benefits of a prebiotic skincare regimen on improving skin barrier integrity, will help clinicians tailor diagnosis and management strategies for their patients with AD, including those with SOC.
Poster Review
AD is a common, chronic, inflammatory skin condition, with almost 20 million adults and over nine million children in the USA estimated to have this disorder in 2024.1 The prevalence of AD and the tendency to develop severe disease are greater in patients with SOC than in White patients;2,3 however, patients with SOC are under-represented in clinical trials for AD.2,4,5 Guidelines on the management of AD in adults have recently been updated, but specific recommendations for patients with SOC with AD are limited.6-8
The pathophysiology of AD is complex and multifactorial, including skin barrier dysfunction, alterations to immune responses, and environmental factors.9 Filaggrin is a key structural protein for skin barrier integrity.10 NMFs are a complex mixture of water-retaining compounds (including amino acids, urea, and lactate) generated by filaggrin degradation that are essential for maintaining skin hydration and skin barrier integrity.11,12
Loss-of-function mutations in the Filaggrin gene are linked to NMF deficiency.13 These mutations have been implicated in skin barrier dysfunction and the development of AD, through an increase in transepidermal water loss, skin pH alterations, and skin dehydration.9,10,11,14 The Filaggrin gene loss-of-function mutations are less common in patients with SOC compared with White patients.3.13 However, the clinical relevance of this disparity across diverse racial/ethnic populations is unclear.13,15
In the context of the unexplained variation in the incidence of AD across different racial/ethnic groups, an exploratory study was conducted to investigate the properties of the skin barrier in patients with AD from diverse racial/ethnic populations who were following a prebiotic skincare regimen as part of a larger study.16 Twenty patients aged 3–80 years with skin phototypes Fitzpatrick I–VI17 and mild AD underwent dermatological evaluations and then followed a prebiotic skincare regimen for 10 weeks. The regimen comprised cleanser (Lipikar AP+ Gentle Foaming Cleansing Oil; La Roche-Posay Laboratoire Dermatologique, Levallois-Perret, France) once a day for 2 weeks, followed by cleanser once a day and moisturizer (Lipikar AP+M Moisturizing Cream; L’Oréal SA, Clichy, France) twice a day for a further 8 weeks. All patients completed the study. Clinical grading and imaging (SkinCam®, QIMA Life Sciences, Gençay, France) were conducted at baseline (Week 0) through Week 10. Skin barrier analyses were conducted using tape-stripped18 skin from the patients’ legs and included NMF analysis as well as corneocyte micromorphology assessment using scanning electron microscopy at baseline and Week 10.
The prebiotic cleanser and moisturizer skincare regimen statistically significantly reduced AD severity and increased skin hydration in normal and lesional skin at Week 4 compared with baseline (p<0.05; Figure 1A and B). These improvements were maintained through Week 10. In addition, skin pH levels were statistically significantly decreased in normal and lesional skin at Week 10 compared with baseline (p<0.05; Figure 1C). These results indicate that a prebiotic skincare regimen reduces disease severity and strengthens skin barrier properties in patients with AD starting from as early as 4 weeks.
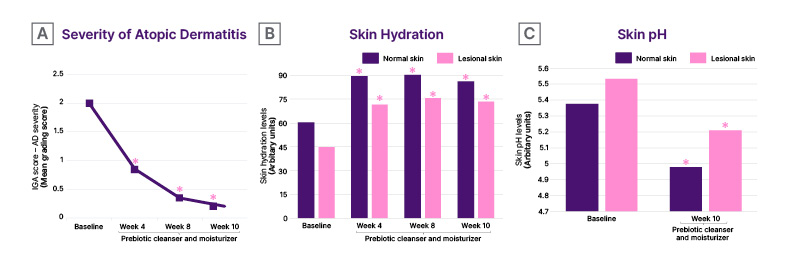
Figure 1: Atopic dermatitis severity, skin hydration, and skin pH in patients with atopic dermatitis following a prebiotic skincare regimen.
*p<0.05 versus baseline.
AD: atopic dermatitis; IGA: Investigator Global Assessment.
The prebiotic skincare regimen also improved skin surface organization patterns in normal and lesional skin. The stratum corneum isotropy total score for corneocytes from baseline to Week 10 increased from 4.20 to 4.90 in normal skin and from 3.85 to 4.75 in lesional skin (both p<0.05). The stratum corneum isotropy total scores for normal and lesional skin were statistically significantly increased for White patients (p<0.05) and showed a trending increase for patients with SOC (data not shown).
Patients with SOC with AD had statistically significantly lower baseline levels of NMF in normal skin compared with White counterparts (28% lower; p<0.05; Figure 2A). The prebiotic skincare regimen statistically significantly increased NMF levels in lesional skin of White patients and patients with SOC by Week 10 (29% and 16% increase, respectively; p<0.05; Figure 2B).
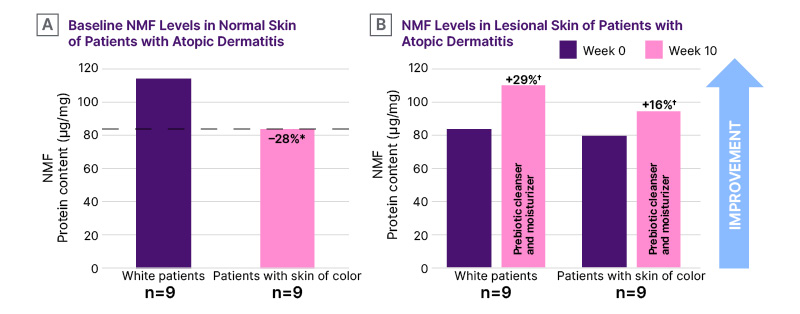
Figure 2: Levels of natural moisturizing factor in normal and lesional skin of patients with atopic dermatitis.
*p<0.05 for patients with skin of color (Fitzpatrick IV–VI)17 versus White counterparts (Fitzpatrick I–III).17
†p<0.05 versus baseline.
NMF: natural moisturizing factor.
Clinical imaging showed visible improvement in AD at Week 10 of the prebiotic skincare regimen in patients from diverse racial/ethnic populations (Figure 3).
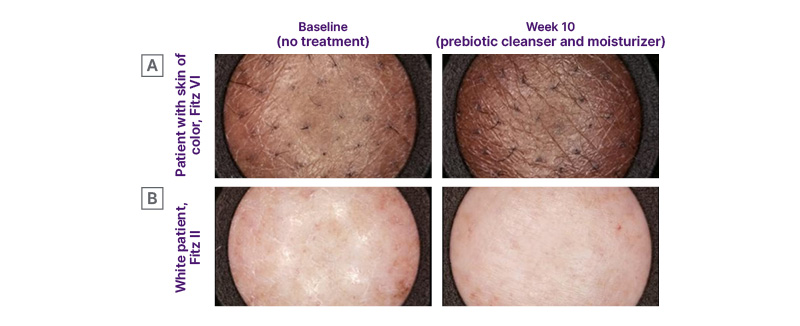
Figure 3: Representative images showing average response in lesional skin of patients with atopic dermatitis at baseline and Week 10 of a prebiotic skincare regimen.16
Adapted from Dumbuya H et al.16
Fitz II: skin phototype Fitzpatrick II;17 Fitz VI: skin phototype Fitzpatrick VI.17
This exploratory study showed that a prebiotic skincare regimen improves AD symptoms in patients from diverse racial/ethnic populations by restoring skin barrier integrity, with improvements reported in disease severity, skin hydration, skin pH, stratum corneum isotropy total score, and NMF levels. Notably, baseline NMF levels were lower in patients with SOC compared with White patients, which may partly explain the differences in AD prevalence and disease severity between these populations. As a knowledge-gathering, exploratory study, the design was open-label and had certain limitations, including a small sample size comprising patients with AD in the less severe range. It would have been interesting to include a comparator, evaluate the effect of the prebiotic cleanser alone versus the prebiotic skincare routine, or compare findings among pediatric versus adult patients presenting with AD. Although informative, it is unclear whether the results can be extrapolated to subjects with moderate or severe AD. Raising awareness of the variation in NMF levels in diverse racial/ethnic populations, as well as the potential benefits of a prebiotic cleansing and moisturizing regimen on improving skin barrier integrity, will help clinicians create tailored diagnosis and management strategies for their patients with AD, including those with SOC.