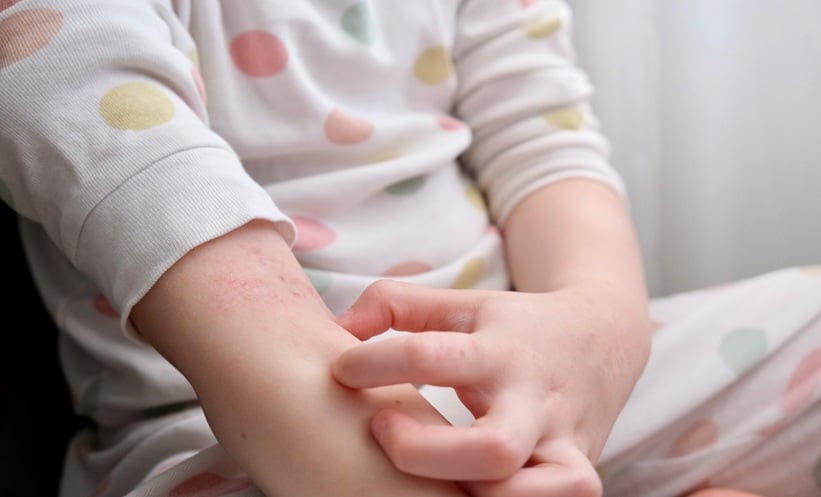
A MULTICENTRE trial has found that maternal prebiotic supplementation during pregnancy does not prevent atopic dermatitis (AD) in infants at high risk of allergic disease. The findings challenge previous assumptions that optimising the maternal gut microbiota could protect offspring from atopic conditions.
The Role of Antenatal Prebiotics in Allergy Prevention
The PREGRALL trial enrolled 376 pregnant women with a history of atopic disease, such as asthma, allergic rhinitis, eczema, or food allergy, across multiple centres. Participants were randomly assigned to receive a daily prebiotic supplement (galacto-oligosaccharides/inulin, n=188) or placebo (maltodextrin, n=188) from 20 weeks of gestation until delivery. Both participants and investigators were blinded to treatment allocation. The primary goal was to determine whether antenatal prebiotic intake could reduce the risk of AD at 1 year in infants considered at high risk. Secondary outcomes included AD severity, quality of life, tolerance of prebiotics, and the prevalence of other atopic diseases.
After one year of follow-up, the results showed no significant difference between the prebiotic and placebo groups. In the intention-to-treat analysis, the odds ratio for developing AD was 1.01 (95% CI 0.59–1.74; P=0.97). Prebiotic supplementation also did not reduce disease severity, improve quality of life, or affect the prevalence of food allergies or allergen sensitisation. Subgroup analyses based on breastfeeding status or delivery mode similarly showed no effect.
Future Directions After Antenatal Prebiotics Trial Results
The authors conclude that maternal intake of GOS/inulin prebiotics during pregnancy does not confer protection against AD in high-risk infants, suggesting that prenatal microbiota modulation alone may not be sufficient to prevent allergic disease. They note that while prebiotics are generally safe and well-tolerated, other preventive strategies should be explored to reduce the rising burden of atopic dermatitis.
This study highlights the importance of rigorous clinical trials in evaluating early-life interventions for allergy prevention. Although preclinical and postnatal studies had suggested potential benefits, the PREGRALL trial indicates that the prenatal window may not be the optimal period for prebiotic intervention to prevent atopic dermatitis. Future research may focus on combining maternal and infant interventions or exploring alternative strategies to support immune tolerance and gut microbiota development in early life.
Reference
Barbarot S. et al. Evaluation of antenatal prebiotic intake in infants at risk of atopy: the double-blind PREGRALL randomized clinical trial. Br J Dermatol. 2025; DOI: 10.1093/bjd/ljaf414