Abstract
The case concerns a 13-year-old male patient admitted to the authors’ department, presenting with the following symptoms: malaise, fever, cough, and scattered vesicular and targetoid skin eruptions on his limbs, earlobe, and trunk, sparing the scalp, palms, and soles. Furthermore, mucositis was observed at three mucosal sites: ocular, oral, and urogenital. The mucosal involvements included bilateral conjunctivitis, stomatitis, initial herpangina-like eruptions on the buccal mucosa and the soft palate, and lip crusts. These subsequently progressed to severe mucositis and ulcerative lesions of the glans penis. Serological testing yielded positive results for IgM antibodies to Mycoplasma pneumoniae and varicella-zoster virus. The chest X-ray indicated atypical pneumonia. The diagnosis was made based on the patient’s medical history, clinical presentation, paraclinical investigations, and serological findings. The treatment regimen was symptomatic, localised, and etiological (oral azithromycin for 5 days, intravenous Ig for 5 days and intravenous acyclovir for 10 days). The patient’s clinical evolution was prolonged, with full recovery taking several weeks.
Key Points
1. Community-acquired pneumonia is the leading cause of hospitalisation and infection-related death, with the most frequent bacterial aetiology being Mycoplasma pneumoniae.
2. This case report presents a reactive infectious mucocutaneous eruption triggered by M. pneumoniae and varicella-zoster Virus, including images, clinical, and paraclinical examinations in chronological order.
3. Reactive infectious mucocutaneous eruption, including M. pneumoniae-induced rash and mucositis, shares a common immune-mediated pathomechanism.
INTRODUCTION
Cases involving co-infections with Mycoplasma pneumoniae and varicella-zoster virus (VZV) are exceedingly rare in medical literature.
M. pneumoniae infections are characterised by a long incubation period (2–4 weeks) and asymptomatic carriage for weeks following infection. Most frequently, it causes upper respiratory tract infections, bronchitis, community-acquired pneumonia (10–30%), and extrapulmonary complications (40%), including mucocutaneous diseases such as erythematous maculopapular/vesicular rashes, urticaria, M. pneumoniae-induced rush and mucositis (MIRM), erythema multiforme, and Stevens-Johnson syndrome. Encephalitis, haemolytic anaemia, and carditis are rare and are associated with respiratory infection.1-4
Extrapulmonary manifestations of M. pneumoniae infection are categorised into direct, indirect, and vascular occlusion mechanisms. The theories suggest MIRM is caused by both direct bacterial effects and indirect immune-mediated processes. The indirect mechanisms include immune modulation via autoimmunity or formation of immune complexes. While M. pneumoniae typically doesn’t infect squamous epithelia, its isolation from blister fluid suggests hematogenous dissemination. The pathogenesis of M. pneumoniae includes multiple mechanisms:1 1) the Community-Acquired Respiratory Distress Syndrome toxin targets NLRP3 inflammasome, resulting in increased production of the pro-inflammatory cytokine IL-1β and enhancing the host innate immune response; 2) hydrogen peroxide and nuclease production of M. pneumoniae also contribute to cellular damage; 3) inflammatory response mechanisms include TLR2 recognition of M. pneumoniae lipoproteins, autophagy-mediated signalling, and activation of inflammasomes, and the cytadherence properties of M. pneumoniae; 4) molecular mimicry between the M. pneumoniae P1 adhesin protein and keratinocyte antigens leads to cross-reacting autoantibodies against mucosal antigens; 5) immune complexes deposited in mucosa and skin can activate the complement system or phagocytic cells; 6) polyclonal activation of B cells and plasma cells at sites of infection leads to altered responses to other unrelated antigens or infections; 7) genetic susceptibility; and 8) combination of infection and medication can trigger mucosal lesions, where interferon and other cytokines downregulate cytochrome P450 enzyme expression, thereby lowering the threshold for a medication to cause a drug reaction.
MIRM is a relatively new entity (introduced in 2015) used to describe mucosal with or without dermal reaction triggered by M. pneumoniae infection.4,5 Ramien et al.6 proposed to the term reactive infectious mucocutaneous eruption (RIME) to unify the terminology for MIRM and other clinically similar mucosal-predominant parainfectious eruptions. MIRM is essentially a subtype of RIME caused by M. pneumoniae. Therefore, in the following sections, the authors will focus on the characterisation of RIME.
RIME represents a broader category of mucocutaneous reactions with an infectious aetiology (including non-M. pneumoniae and viral causes), but it does not encompass toxic or iatrogenic aetiologies. Diagnosis of RIME requires evidence of an infectious trigger, including a preceding history (7–10 days) of cough, fever, malaise, or arthralgia, supported by clinical examination or investigations confirming a respiratory infection. Diagnostic evaluations include chest radiography and laboratory testing for acute infections caused by respiratory viruses, M. pneumoniae, or Chlamydophila pneumoniae. These assessments may involve culture or PCR analysis of nasopharyngeal/oropharyngeal samples, or serological testing for IgM and acute/convalescent IgG. Confirmation of RIME requires at least two of the following criteria: a non-contributory medication history; erosive mucositis affecting two or more sites; and vesiculobullous lesions or atypical (often bullous) target lesions covering <10% of the body surface area. Supporting features include prodromal symptoms and histological findings excluding alternative diagnoses.7
PATIENT INFORMATION
A 13-year-old male with an unremarkable medical history except for a negative history for chicken pox, no prior vaccination for VZV, and a four-year history of athletic activity. The patient was unable to recall any contact with an ill individual within the past month.
The patient’s first presentation at the emergency department was on the 8th day post-onset of an acute illness. The patient exhibited symptoms of malaise, non-productive cough, bilateral conjunctivitis (without chemosis), stinging ocular pain, crusted lips, herpangina-like eruptions on the buccopharyngeal mucosa (Figure 1). The lung auscultation revealed diffuse crackles sounds; and a chest X-ray was performed, which indicated atypical pneumonia (right middle lung field cuffing; Figure 2A).
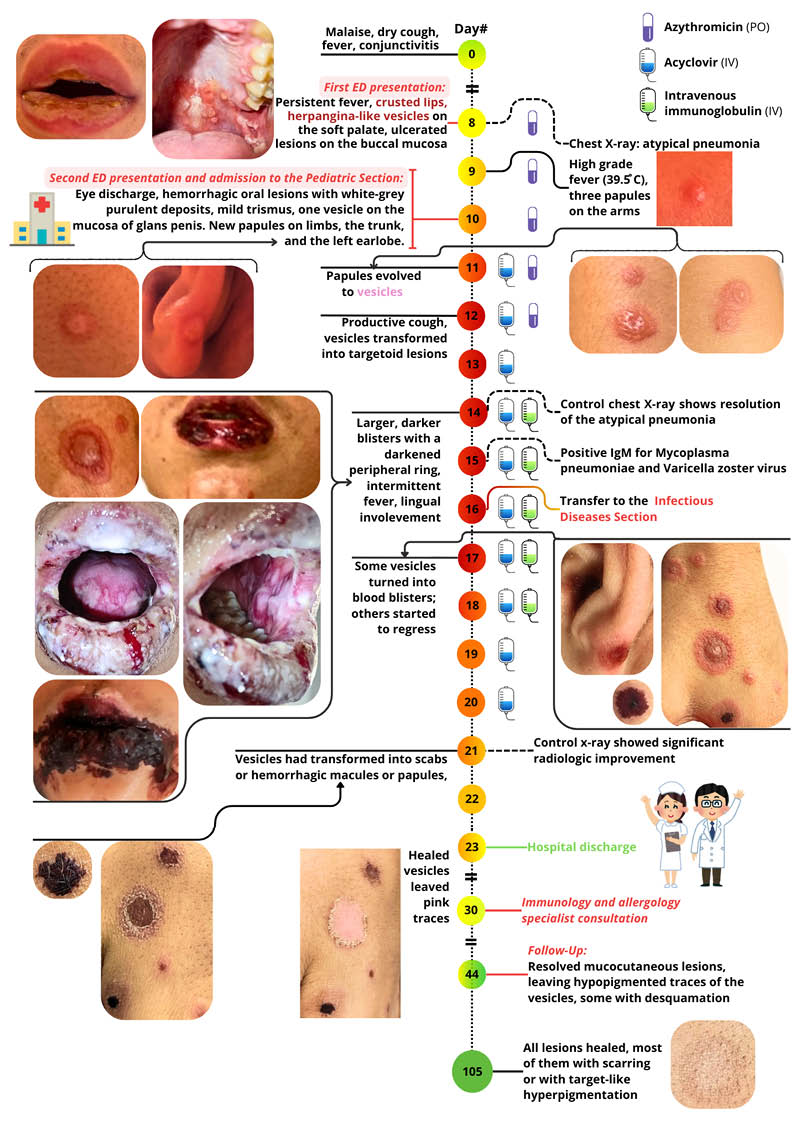
Figure 1: Clinical timeline.
ED: emergency department; IV: intravenous; PO: oral.
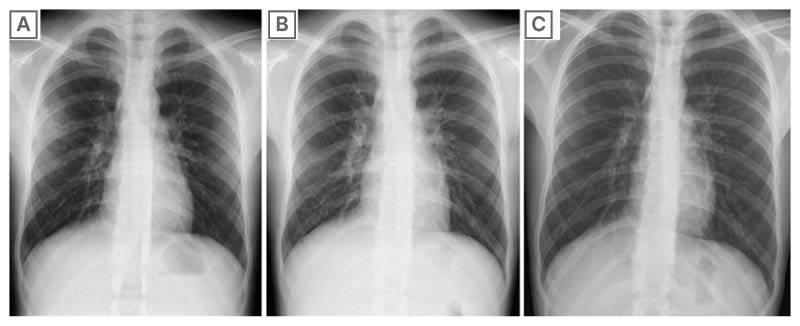
Figure 2: The patient’s X-ray results from Day 8, 14, and 21.
2A) Day 8: pulmonary radiography described right middle lung field cuffing and bilateral hilar prominence. 2B) Day 14: control radiography described right-sided accentuated hilar and perihilar prominence, with resolution of the opacity in the right upper lobe. 2C) Day 21: control radiography showed significant radiologic improvement, with mild bilateral hilar, perihilar, and infrahilar prominence.
The patient was discharged with the following prescription: azithromycin 500 mg orally once daily for 5 days; Anaftin spray (Ketof, Salutas Pharma GMBH, Germany; 15 mL, three puffs four times/day) contains polyvinylpyrrolidone and hyaluronic acid; salbutamol sulphate inhaler; an expectorant; an antitussive; ibuprofen and paracetamol in case of fever.
Following a 2-day interval, the patient re-presented to the emergency department and was subsequently admitted to the paediatric section with a presumptive diagnosis of Stevens-Johnson syndrome, induced by the non-steroidal anti-inflammatory drug (ibuprofen).
Clinical Findings at Admission
Physical examination revealed high-grade, refractory fever (39.5 °C/103.1 °F), scattered papulovesicular eruptions with erythematous halos on the arms and trunk, sparing the scalp, palms, and soles. Furthermore, a vesicle was observed on the glans penis. On the swollen lips and buccopharyngeal mucosa, haemorrhagic and buccal ulcers with white-grey purulent deposits were noted, accompanied by mild trismus due to pain. Notably, there was an absence of arthralgia, hepatosplenomegaly, and lymphadenopathy.
TIMELINE
On the 11th day of the disease’s progression, the generalised exanthem extended to the left auricle and evolved into vesicles resembling those seen in varicella. The vesicles were slightly larger, reducing the likelihood of Stevens-Johnson syndrome. Intravenous acyclovir therapy was initiated.
On the 12th day, the vesicles transformed into targetoid lesions; their contents were clear. The lip lesions became haemorrhagic and crusted. The patient developed a productive cough with pink sputum, intermittent fever (a diffuse, generalised dermal erythema developed while the fever was present). The conjunctivitis resolved.
On the 13th day, suspicion was raised for Kawasaki disease. However, as not all criteria for Kawasaki disease were present, intravenous Ig therapy was started the next day.
On the 14th day, some vesicles evolved into larger, darker blisters with a darkened peripheral ring. The oral mucosa became extremely painful due to the oral ulcers, haemorrhagic crusting, and the patient could not feed. The control X-ray described right-sided accentuated hilar and perihilar prominence, and resolution of opacity in the right upper lobe (Figure 2B).
On the 15th day, serological testing (chemiluminescence immunoassay) yielded positive results for IgM antibodies to M. pneumoniae and VZV. The herpes simplex virus 1+2 IgM antibodies were negative.
On the 16th day, the patient was transferred to the infectious disease section. He was afebrile. Aspirin was stopped, as it can lead to Reye’s syndrome in varicella.
On the 17th day, some vesicles turned into blood blisters, while others started to regress. A paediatric cardiology consultation ruled out the cardiac complications associated with Kawasaki disease. Consequently, intravenous Ig therapy was stopped after a total of 5 days of treatment.
On the 21st day, most vesicles had transformed into scabs or haemorrhagic macules or papules. Some vesicles healed, leaving pink traces. The control X-ray showed significant radiological improvement, with mild bilateral prominence of the hilar, perihilar, and infrahilar regions (Figure 2C).
On the 23rd day, due to the extent of healing of the oral, urogenital mucosa, and the skin lesions, and as the patient no longer had a fever, he was discharged from the hospital with symptomatic treatment.
DIAGNOSTIC ASSESSMENT
The diagnosis of RIME due to coinfection with M. pneumoniae and VZV was established based on the integration of clinical presentation, laboratory findings, and imaging results. The illness began with a prodromal phase, characterised by high fever and cough, subsequently progressing to atypical pneumonia. Physical examination identified a rash that evolved into papules, vesicles, target-like eruptions affecting <10% body surface area, with involvement of three mucosal sites: conjunctival, oral, and urogenital. Laboratory results indicated the presence of leucocytosis, neutrophilia, lymphocytopenia, and elevated acute phase protein levels. Serological tests were positive for IgM antibodies to M. pneumoniae and VZV, therefore the authors think RIME is a more accurate diagnosis than MIRM with VZV co-infection.
The diagnostic process was complicated by the parents’ refusal to consent to vesicle sampling or skin biopsy; the serology results took too long to arrive, and the unavailability of PCR testing as a diagnostic tool further delayed the establishment of the diagnosis.
TREATMENT
Etiologic treatment:
- Azithromycin 500 mg orally once daily for 5 days
- Acyclovir 250 mg intravenous (100 mL/min) four times daily for 10 days
- Privigen (intravenous Ig, CSL Behring GMBH, Germany) 2.5 g intravenous (25 mL/h) once daily for 5 days.
Local agents:
- Oral mucosa: Anaftin spray (15 mL, three puffs four times/day); mouthwash preparation containing borax glycerine, nystatin, anestezine, metronidazole, vitamin A; chlorhexidine digluconate mouthwash (three times/day); bathing with sage tea.
- Conjunctival mucosa: netilmicin+dexamethasone eye drops (four times/day) and artificial tear in both eyes.
- Genital mucosa: pantothenic acid cream, chlorhexidine digluconate.
Supportive therapy: Paracetamol 1 g intravenous three times/day; aspirin 500 mg orally four times/day; salbutamol sulphate inhaler; expectorant; an antitussive (Ketof); multivitamins; inosine pranobex 500 mg orally twice/day for 10 days.
FOLLOW-UP AND OUTCOMES
An allergology and immunology consultation was conducted, and laboratory tests were performed. All results were within normal range except where indicated in bold (see Supplementary Table 1): complete blood count, AST, ALT, serum albumin, ALP, urea, creatinine, total IgA, IgM, IgG, CRP, complement C3c, C4, IL-6, circulating immune complexes, ANA screen, anti-cardiolipin IgG Ab, ACA IgG Ab, anti dsDNA IgG, anti Jo-1 IgG, anti MPO-ANCA IgG, anti nRNP or Sm IgG, anti SCL-70 IgG, Anti Sm IgG, Anti SS-A IgG, Anti SS-B IgG, B2 glycoprotein 1 IgG, anti HSV-1 and 2 IgM and IgG, anti VZV IgM and IgG, anti SARS-CoV2 IgM, and anti M. pneumoniae IgM and IgG.
Re-evaluation by the infectious diseases unit showed favourable progression, as evidenced by the resolution of cutaneous lesions with residual mild hypopigmentation. The oral and urogenital mucosa were clear, with minimal hyperaemia. Laboratory investigations revealed eosinophilia and absence of inflammatory markers.
DISCUSSION
Strengths
The strengths of the case presentation lie in its well-photodocumented, chronologically structured format, which encompasses comprehensive clinical and paraclinical examinations, along with interprofessional consultations in dermatology, ophthalmology, paediatric cardiology, aimed at excluding other diseases. This method ensures a systematic and evidence-based approach, facilitating diagnostic accuracy and enhancing the clarity of findings.
Limitations
Although PCR testing for viral DNA is the recommended method for diagnosing varicella, limited availability restricts diagnostic options. In the authors’ case, despite the unavailability of PCR testing, serological analysis conducted at two distinct time points confirmed a recent varicella infection. The initial serological result was positive for varicella-specific IgM antibodies but negative for IgG. An additional limiting factor was the absence of parental consent for obtaining vesicular fluid samples for further analysis. It’s important to note that PCR is a more sensitive diagnostic method compared to serological testing, meaning that enzyme immunoassay tests with lower sensitivity may yield false-negative results. Park et al.8 reported that the sensitivity and specificity of PCR analysis of salivary DNA for detecting VZV were 88% and 100%, respectively.8 Kombe Kombe et al.9 found that the sensitivity of chemiluminescence immunoassay-based detection for IgM and IgG antibodies in varicella was 93.6% and 69.4%, respectively, while the specificity was 98% and 99.8%, respectively.9
The diagnostic process for RIME was notably complex due to the overlapping clinical features with VZV infection. Although VZV can cause RIME or erythema multiforme, it is a rare occurrence compared to herpes simplex virus. M. pneumoniae is known to cause both erythema multiforme and MIRM.1,10,11
Comprehensive examinations excluded other conditions such as Kawasaki disease, Stevens-Johnson syndrome, toxic epidermal necrolysis, co-infections (HIV, syphilis), and autoimmune diseases (lupus, rheumatoid arthritis, scleroderma, antiphospholipid syndrome, systemic sclerosis, polymyositis, dermatomyositis, vasculitis, mixed connective tissue disease, Sjögren’s syndrome). All tests returned negative results.
CONCLUSION
This case presentation aligns with recent academic studies on RIME, particularly regarding symptom manifestation, clinical and paraclinical findings. In an adolescent male patient, following a prodromal phase of general symptoms, lesions appeared on three mucosal surfaces: oral, ocular, and urogenital, accompanied by maculopapular vesicular skin eruptions. These later evolved into target-like lesions, which were sparsely distributed across the skin while sparing the face, scalp, palms, and soles.
Although PCR is the gold standard for determining the aetiology of VZV, both PCR and serology are recommended for diagnosing M. pneumoniae in paediatric patients.1 English-language medical literature contains limited studies on VZV-induced RIME. However, two documented cases describe VZV reactivation following SARS-CoV-2 coinfection with influenza A and coronavirus NL63 co-infection with group A Streptococcus. In both cases, mucosal involvement was reported; however, only the paediatric patient exhibited rash affecting 40–50% of the body surface area, later progressing to 70%. The VZV DNA PCR test was positive, confirming viral presence in multiple erosions.12,13 The fact that RIME can be triggered by a diverse range of infectious agents supports the theory that VZV induces RIME through an indirect rather than a direct mechanism. While antiviral therapy for VZV might be necessary to treat an underlying infection, immunomodulatory agents are likely crucial for controlling the mucocutaneous eruption itself.
Future studies are warranted to better understand the pathophysiology and optimal treatment strategies for MIRM, RIME, and similar conditions.







