Abstract
Leiomyosarcoma (LMS) of the inferior vena cava (IVC), although rare, is the most common primary tumour of the IVC. Due to a vague, non-specific clinical presentation, radiological investigations play an important role in diagnosis. The tumours are classified based on the segment of the IVC involved and the growth pattern. The authors report a case of a 41-year-old man who presented with complaints of pain in the right hypochondrium, abdominal distension, and occasional episodes of fever for 1 month. Imaging features showed a well-defined, lobulated, heterogeneous lesion in the retroperitoneum invading the IVC, causing its expansion. There was an extension into the right atrium. Histopathological findings confirmed the diagnosis of an LMS. Surgery is the mainstay of treatment for such tumours. This case highlights the imaging features of a retroperitoneal LMS involving the IVC, causing its expansion and extension into the right atrium.
Key Points
1. Inferior vena cava leiomyosarcoma (iLMS) is a rare but aggressive tumour with a high rate of metastasis (around 50%). It is crucial to differentiate iLMS from tumours with secondary inferior vena cava involvement due to differences in pathology.2. This article presents a case report of a 41-year-old man with iLMS, emphasising imaging findings and the role of multiparametric MRI/CT in diagnosis.
3. The reporting checklist should include the segment of inferior vena cava involved, presence of collaterals, extent of the lesion, and adjacent organ invasion. Prompt use of radiological investigations, such as MRI, is a must for appropriate diagnosis.
INTRODUCTION
Leiomyosarcoma (LMS) is a malignant neoplasm characterised by smooth muscle differentiation. It is the second most common sarcoma to affect the retroperitoneum and the most common sarcoma to arise from the retroperitoneal large blood vessels.1 LMS of the inferior vena cava (IVC) or IVC leiomyosarcoma (iLMS) accounts for 2% of all LMSs and originates from the smooth muscle cells of the media.2 It is most commonly seen in women and frequently occurs in the sixth decade of life.3 Based on the segment involved, it can be classified into segment I (infrarenal), segment II (renal and suprarenal), and segment III (suprahepatic). Segment II is the most common type.4,5 Based on the growth pattern, it can be either extraluminal (most common), intraluminal, or a combination of both.5-7 Since it is a rare tumour, it is important to differentiate it from the other common tumours with secondary involvement of the IVC, given the poor prognosis and high rates of recurrence. The need to differentiate iLMS from other organ tumours with secondary IVC involvement is described in detail in the discussion section.
CASE PRESENTATION
A 41-year-old man presented with complaints of pain in the right hypochondrium with abdominal distension for 1 month. He had a low-grade fever intermittently for 1 month. On examination, a lump was palpable in the right hypochondrium and the right lumbar region. Blood investigations such as white blood cell counts, liver enzymes, and bilirubin were in the normal range. He had no relevant past medical, surgical, or family history. On ultrasound (Figure 1), there was a well-defined, lobulated, heterogeneously hypoechoic lesion in the retroperitoneum on the right side. The lesion involved the IVC, causing distension. On colour Doppler, there was vascularity within the lesion. The IVC showed an absence of colour flow. An unenhanced CT was ordered to map the extent of the lesion, since the serum creatinine levels were elevated. CT showed a heterogeneous soft-tissue density lesion in the right retroperitoneum measuring approximately 6.5x11x25 cm. It was separate from the kidney, the adrenal gland, and the pancreas. There was no macroscopic fat or calcification. There was loss of fat planes with the infrarenal IVC, with significant dilatation of its proximal segments (imperceptible wall sign). Superiorly, it extended into the right atrium (Figure 2). A few days later, after the creatinine levels dropped to normal limits, a contrast-enhanced MRI scan was done for further characterisation of the lesion. MRI was preferred over a repeat contrast-enhanced CT, as MRI has superior contrast resolution and lacks ionising radiation. The lesion was heterogeneously hyperintense on the T2 sequence and isointense on the T1 sequence. There was no signal drop on the in-phase and out-of-phase sequences. Post-contrast sequences showed heterogeneous enhancement. The lesion showed diffusion restriction with a drop in the apparent diffusion coefficient map (Figure 3). There was involvement of the right ureter with resultant proximal dilatation. A percutaneous CT-guided biopsy of the lesion showed a spindle cell tumour with moderate cellular atypia. On immunohistochemistry, the cells showed strong positivity for desmin, smooth muscle actin, and h-caldesmon. They tested negative for S-100, discovered on GIST1 (DOG1), and mouse double minute 2 (MDM2). The patient was planned for neoadjuvant chemotherapy to downsize the tumour prior to surgery; however, he was lost to further follow-up.
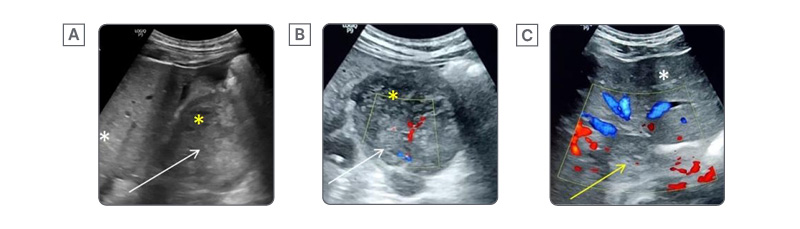
Figure 1: Ultrasound and colour Doppler findings in a case of inferior vena cava leiomyosarcoma.
A) Longitudinal sections of the ultrasound of the abdomen show a heterogeneous lobulated lesion in the retroperitoneum (white arrow) with areas of necrosis (yellow asterisk). The white asterisk marks the liver.
B) and C) Axial sections on colour Doppler show vascularity within the lesion (white arrow). Necrotic areas (yellow asterisk) show no vascularity. The IVC is distended with no colour flow, indicating a tumour thrombus (yellow arrow). The white asterisk marks the normal liver.
IVC: inferior vena cava.
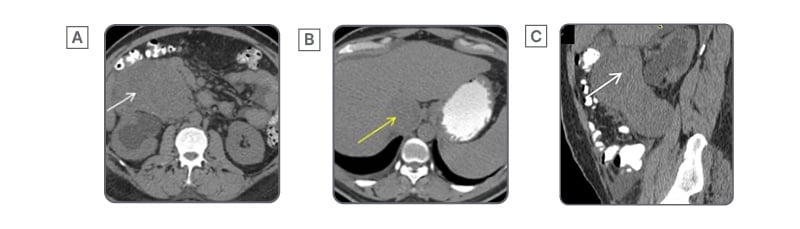
Figure 2: Imperceptible wall sign of inferior vena cava leiomyosarcoma.
Axial (A, B) and sagittal (C) sections of an unenhanced CT abdomen show a well-defined, heterogeneous, soft tissue lesion (white arrow) in the retroperitoneum. There is loss of fat planes with the IVC, with its distension due to tumour thrombus (yellow arrow), demonstrating the imperceptible wall sign.
IVC: inferior vena cava.
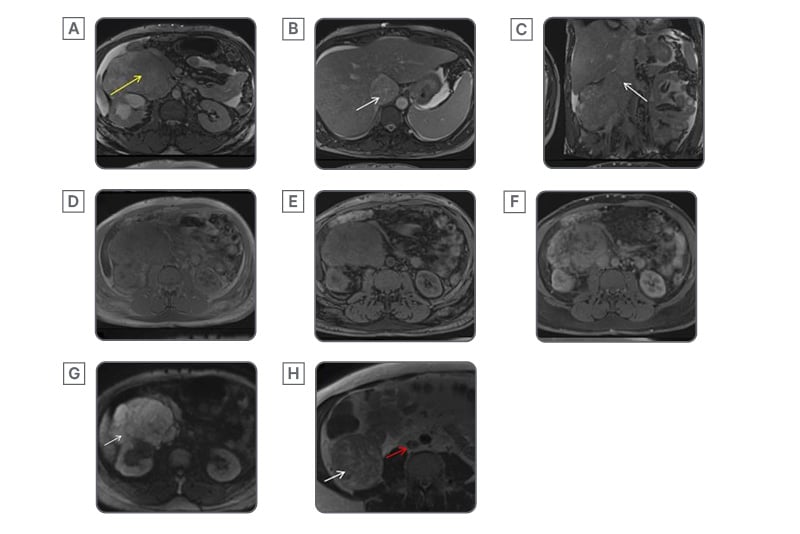
Figure 3: MRI features of inferior vena cava leiomyosarcoma.
Axial (A, B) and coronal (C) T2 sequences of the MRI abdomen show the lesion to be hyperintense (yellow arrow). There is a loss of fat planes surrounding the IVC, causing distension (white arrow) and extending to the right atrium. Axial pre-contrast T1 in-phase (D) and out-of-phase (E) sequences show no signal drop. Axial post-contrast T1 sequence (F) shows heterogeneous enhancement. Axial diffusion-weighted sequence (G) shows diffusion restriction (white arrow). Axial non-fat saturated T2 sequence of the MRI of the abdomen (H) shows the intact IVC (red arrow) below the level of the tumour. The white arrow marks the extraluminal component of the tumour in (H).
IVC: inferior vena cava.
DISCUSSION
Retroperitoneal LMSs typically present as large soft tissue masses. Due to their retroperitoneal location and their tendency to spare visceral structures, these tumours can grow to substantial sizes before detection and are often incidentally discovered during imaging.3 When symptomatic, they may cause compression-related symptoms, such as pain. If they have an intraluminal component, they tend to present earlier.8 Based on the involved segment of the IVC, the presentation may vary. Signs of Budd-Chiari syndrome, such as jaundice, hepatomegaly, ascites (suprahepatic IVC), renal dysfunction (mid IVC), or limb oedema (infrarenal IVC), may be present.2,6
Imaging plays an important role in differentiating iLMS from adjacent organ tumours.9 On ultrasound, these tumours are typically large, solid, soft-tissue masses with lobulated margins, often with cystic areas secondary to necrosis or haemorrhage.10 They may be iso- or hypoechoic compared with the liver. An intravascular growth pattern shows an intraluminal solid mass with vascularity on Doppler. The IVC is often expanded.
On CT, these tumours are isoattenuating to muscle. The enhancement pattern is heterogeneous. Collateral vessels are frequently observed adjacent to the lesion due to the slow growth of the tumour.11,12 Local invasion into the right kidney, liver, adrenal gland, pancreas, stomach, or spine is common. The intravascular component is seen as a tumour thrombus. CT provides certain signs that might help in differentiating the lesion from a primary retroperitoneal tumour. The imperceptible wall sign is the most specific, wherein the vessel is not seen separate from the lesion at the site of maximal contact, as seen in the authors’ case.11 Other signs, such as the positive embedded sign (the vessel is embedded in the periphery of the lesion), might be encountered.13,14 The negative embedded sign, indicating vessel compression at the periphery of the lesion, suggests that the lesion does not originate from the vessel.11 The extraluminal variant is difficult to diagnose and has to be differentiated from other tumours secondarily involving the IVC.5 Tumours known to invade the IVC include renal cell carcinoma, Wilms tumour, and adrenal cortical carcinoma. These tumours have a primary tumour arising from the parent organ, whereas a primary iLMS will lack this finding.
On T2 sequences, the lesion has a heterogeneously hyperintense signal, with a hypo- or isointense signal on T1 sequences.6,10,15 Areas of necrosis are hyperintense on T2 and hypointense on T1. Haemorrhagic areas show blooming on gradient sequences and high signal on T1, which does not suppress on fat-saturated sequences, differentiating it from fat. Due to high cellularity, they show true diffusion restriction.16 In cases with intravascular tumour, black-blood imaging sequences nicely depict the extent of the tumour by highlighting the bright tumour against the dark blood in the vessel. Multiplanar post-contrast imaging is also useful for evaluating vascular involvement and distinguishing tumour from bland thrombus. The radiological findings have been summarised in Table 1.
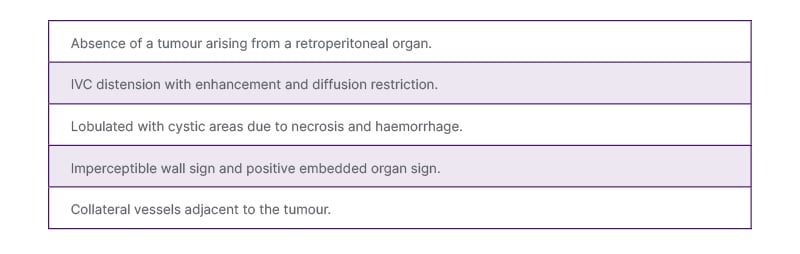
Table 1: Key imaging features of inferior vena cava leiomyosarcoma.
IVC: inferior vena cava.
Certain imaging findings that favour a better prognosis include tumour location in the mid-segment of the IVC. Other favourable factors include pain at presentation (early detection). On the contrary, IVC occlusion, lower limb oedema, and involvement of the upper segment have a poorer prognosis. Intimal sarcomas have a worse prognosis than mural sarcomas due to their propensity for dissemination.9
Primary retroperitoneal tumours are a common differential diagnosis for the extraluminal type; however, in these cases, the IVC is usually displaced or compressed. Bland thrombus does not distend the lumen, lacks diffusion restriction, and is non-enhancing. Certain tumours with tumour thrombosis mimicking iLMS include renal, adrenal, or hepatocellular carcinoma.6 The epicentre of the lesion, along with the characteristic imaging features, helps to eliminate these differentials. The most common primary retroperitoneal tumours include liposarcoma (fat component), lymphoma (uniform homogeneous enhancement, T2 dark with strong diffusion restriction, and encasement of vessels), and metastases (multiplicity with the presence of a primary tumour).
Accurate diagnosis is of paramount importance, as vascular LMS is notorious for recurrence at distant sites, with a metastatic rate of almost 50%.9 Differentiation from a solid organ tumour with secondary involvement of the IVC is necessary for the following reasons:
• Pathobiology: iLMS, being mesenchymal in origin, has a separate regimen for systemic therapy, as opposed to an epithelial or mesenchymal tumour of an adjacent organ, which requires an organ-specific regimen.
• Prognosis: iLMS has a high rate of recurrence, even with R0 resection, while the recurrence rate for other tumours is highly variable and depends on the primary organ involved.
• Surgical management: iLMS warrants en bloc resection of the involved IVC segment ± graft reconstruction, whereas in cases of secondary involvement of the IVC, an organ-directed resection is preferred (such as radical nephrectomy/adrenalectomy). The vessel wall is preserved unless invaded.
• Biopsy: For iLMS, a percutaneous transhepatic or retroperitoneal approach is preferred to avoid peritoneal seeding. For tumours with secondary involvement of the IVC, the sample is obtained from the primary organ mass instead of the IVC tumour thrombus (safer with a better diagnostic yield).
Surgical resection is the only curative option.2,17 Sternotomy, along with laparotomy, might be required for segment III tumours. In cases of involvement of the renal or suprarenal veins, there might be a need for right nephrectomy/adrenalectomy. This involves a multidisciplinary team including the surgical oncologist, vascular surgeon, and anaesthesiologist. If only the renal vein ostium is involved, a renal vein reimplantation is done.18 The vessel is reconstructed by ligation or grafts; however, they are not required in the presence of adequate collaterals.4,9 When the tumour is bulky, a multi-organ autotransplantation is performed.12 The use of chemoradiotherapy has been described in both neoadjuvant and adjuvant settings. However, there is no standard regimen.6 Doxorubicin with trabectedin was found to increase the progression-free survival in patients with metastatic or unresectable disease.9
The authors’ case had a non-specific presentation of abdominal pain and distension with on-and-off fever. Thus, the diagnosis was primarily achieved on imaging, which depicted the large, voluminous nature of the tumour with both intra- and extraluminal components, which is a rare finding.2,16 This highlights the importance of radiological investigations and the guidance they offer for surgical management. The radiological pointers relevant to healthcare professionals include the exact extent of the lesion, the segment of the IVC involved, the status of the renal veins, adjacent organs, and the presence of collaterals. These factors allow the clinicians to offer proper counselling to the patient.
CONCLUSION
iLMS is a rare retroperitoneal tumour with a non-specific clinical presentation. Accurate diagnosis is important, as it carries a poor prognosis with a high recurrence rate. Differentiating iLMS from other tumours is also important in terms of systemic therapy regimen, route of biopsy, and surgical management. Radiological signs that aid differentiation from primary retroperitoneal tumours include the imperceptible wall sign and the positive embedded sign. The intraluminal form causes distension of the vessel with heterogeneous post-contrast enhancement. It is important to mention the segment of the vessel involved. MRI should be ordered whenever possible for better characterisation and staging of the tumour. Surgical management is challenging and involves resection of the involved IVC segment, along with its reconstruction. Additional nephrectomy or adrenalectomy may be needed in some patients.