Meeting Summary
Urological cancers are malignancies that affect the organs of the urinary system and male reproductive system. Of these, bladder cancer is the sixth most common cancer in the USA, with 75% of cases being non-muscle invasive bladder cancer (NMIBC). Surgical treatment for urological cancers often lacks durability, requiring multiple procedures due to disease recurrence or progression. New therapies are required to improve treatment prospects, reduce recurrence risk, and maintain patients’ quality of life.
A range of poster presentations took place as part of the American Urological Association (AUA) Annual Meeting 2025. Posters highlighted the potential of reverse thermal gel formulations of anti-cancer agents, including mitomycin as an effective alternative to repetitive surgeries for treating different forms of low-grade urothelial cancers, and zalifrelimab for the treatment of recurrent non-muscle invasive bladder cancer (NMIBC). The posters also considered Patient-Reported Outcomes (PRO) of NMIBC and the effects of hydrogel-based chemotherapy on PROs.
Presenters discussed ongoing research to further evaluate the safety and efficacy of investigational therapies, with the hope of providing an alternative to surgery for patients with NMIBC, as has been demonstrated with low-grade-upper tract urothelial cancers (LG-UTUC).
Introduction
Urological cancers are malignancies that affect the organs of the urinary system and male reproductive system. Of these, bladder cancer is the sixth most common cancer in the USA,1 with 75% of cases being NMIBC.2,3 Despite available treatments, many patients experience disease recurrence or progression, which occurs in 15–30% of high-grade cases and over 50% of intermediate-risk cases.4 New therapies are required to improve treatment prospects, reduce recurrence risk, and maintain patients’ quality of life (QoL).
Current Therapeutic Landscape: Surgical Interventions
Transurethral resection of bladder tumour (TURBT) surgery is the current standard of care for low-grade intermediate-risk NMIBC (LG-IR-NMIBC).5,6 TURBT is associated with local and systemic side effects including haematuria, urinary tract infection (UTI), bladder perforation, and transient increase in urination frequency or urgency. Furthermore, TURBT is performed under anaesthesia with associated pre-operative risks.5,6 Recurrence rates of NMIBC after TURBT are high, with patients often requiring multiple procedures. Around 50% of patients with IR-LG-NMIBC experience disease recurrence within 5 years.7
Similarly, LG-UTUC can be treated with endoscopically guided ablation, but recurrence is common, with a 5-year relapse-free survival rate of only 13–54%.8 Patients therefore require lifelong surveillance in the form of repeat ureteroscopy, with 80% of surveyed healthcare professionals (HCP) choosing to perform a repeat procedure 3 months after the initial surgery.9
While immediately effective, surgical ablation is not typically durable, highlighting the clinical urgency for improved, long-lasting treatments for bladder cancer.
Symptom Burden and Quality of Life in Patients with Urological Cancers
Symptom burden and health-related QoL are critical concerns for patients with NMIBC, further highlighting the need for ongoing improvements to care. The burden of symptoms for bladder cancer patients includes urinary issues, bloating and flatulence, malaise, intravesical treatment issues, and future worries.10 Loss of sexual function and enjoyment are also key factors, including issues around sexual intimacy and perceived risks of contaminating a sexual partner with treatment-related substances.11 These key themes are captured in the European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire (QLQ) for use in patients with NMIBC 2024 (EORTC QLQ-NMIBC24).11 With the EORTC QLQ-NMIBC24, patients numerically score their perception of symptom burden, with a recall of 1 week for most items and 4 weeks for sex-related questions. Patients answer questions including: “Was it difficult for you to get enough sleep, because you needed to get up frequently at night to urinate?” with a scale from 1 (not at all) to 4 (very much). Functional and symptom scores are linearly transformed to a 0–100 scale, with higher symptom scores indicating greater symptom burden, and higher functional scores reflecting better functioning or satisfaction.
Validation of EORTC Quality of Life Questionnaire NMIBC24
Charles Peyton, Professor of Urologic Oncology at the University of Alabama, Birmingham, USA, described how this tool has not yet been validated in patients with LG-IR-NMIBC, and presented new data determining the questionnaire’s psychometric properties with LG-IR-NMIBC patients in global trials:12 the ATLAS13 and ENVISION14 studies.
The ATLAS Study
The ATLAS study13 was a Phase III randomised controlled trial assessing the efficacy and safety of intravesical mitomycin (a reverse thermal hydrogel containing mitomycin) with or without TURBT versus TURBT alone. The trial included patients with newly diagnosed or recurrent LG-IR-NMIBC randomised to six once-weekly intravesical instillations of mitomycin with (n=140) or without (n=142) TURBT.
The ENVISION Study
The ENVISION study14 was a multinational, open-label, single-arm Phase III trial evaluating the efficacy and safety of intravesical mitomycin therapy in patients with recurrent LG-IR-NMIBC. In this trial, patients with recurrent LG-IR-NMIBC received six once-weekly intravesical instillations of mitomycin (n=240). For detailed safety and efficacy results from the ENVISION trial, see section: The ENVISION Trial Results and Long-Term Follow-Up.
For the psychometric validation, EORTC QLQ-NMIBC24 data from ATLAS and ENVISION trials administered at baseline, Week 6, and Month 3 were used. Test-retest ability, internal consistency, known group validity, and sensitivity to change were evaluated. The between-group minimal clinically important difference (MCID) was also assessed.
Results presented at AUA 2025 support the utilisation of EORTC QLQ-NMIBC24 to assess health-related QoL in individuals with LG-IR-NMIBC. Peyton reported a moderate-to-high test-retest reliability measured by intraclass correlations, demonstrating stability of the measurement over time. Where symptom areas were covered by multiple questions, the results of these questions were accordant. This internal consistency was measured by Cronbach’s alpha, which measured >0.75 in all multi-question domains except for malaise. The questionnaire was responsive to changes in health over time, specifically reporting sensitivity to change in urinary symptoms, sexual intimacy, and malaise. Results indicate that the EORTC QLQ-NMIBC24 performed well on known group validity where patients with high scores (>90) on the physical function scale of the QLQ-C30 reported fewer symptoms in NMIBC24 as expected.
MCID data were also presented.12 At Months 3 and 6, between-patient estimates of MCID ranged from 4.37–19.84 and 4.37–19.84, respectively. Importantly, Peyton noted that the MCID estimates reported in these analyses could aid in the clinical interpretation of health-related QoL in patients with LG-IR-NMIBC, thus enabling clinicians to evaluate the impact of different treatment interventions.
PROs are vital when evaluating existing and emerging treatments, alongside safety and efficacy. Peyton highlighted how health-related QoL data can inform both patients and HCPs about the impact of treatment on patient QoL, and indicated that QoL questionnaires such as the EORTC QLQ-NMIBC24 should be used to supplement decision-making both in clinical trials and during patient-HCP discussions.
Considering Patient-Reported Outcomes of Hydrogel-Based Chemotherapy for Non-muscle Invasive Bladder Cancer
With symptom burden and health-related QoL being critical concerns for bladder cancer patients,10 investigating the impact of emerging therapies on QoL is key. At AUA 2025, Peyton presented a poster detailing PROs following treatment of LG-NMIBC with hydrogel-formulated intravesical mitomycin across three clinical trials: ATLAS (n=142),13 ENVISION (n=240),14 and OPTIMA II (n=63).15,16
The OPTIMA II Study
The OPTIMA II study was an open-label, single-arm, Phase II trial with patients recruited from 20 sites in the USA and Israel after a positive biopsy result for LG-IR-NMIBC.15 A total of 63 patients were treated with six once-weekly instillations of mitomycin intravesical solution (1.33 mg/mL), and 57 patients received all six doses. The primary outcome was complete response (CR), which was defined as visual confirmation and negative wash urine cytology and for-cause biopsy. At the 3-month primary endpoint evaluation, 65% of patients (n=41) patients achieved CR. Of these, 95% (n=39), 73% (n=30), and 61% (n=25) of patients remained disease-free at 6-, 9-, and 12-months post-treatment, respectively. Common adverse events with an incidence of ≥10% included an increase in urinary frequency and urgency, dysuria, haematuria, UTI, and fatigue.
Patient-Reported Outcomes Following Intravesical Mitomycin Treatment
PROs were evaluated using the previously validated12 EORTC-QLQ-NMIBC24 at baseline, 3 months, and 12 months. Peyton highlighted from the ENVISION study that 80% of patients treated with intravesical mitomycin achieved a CR at 3 months, with an 82% probability of remaining in response 12 months later.14.16
Patients enrolled in the three studies were predominantly male (65%) and had a median age of 69 years. At baseline, patients reported a high level of functioning and low symptom burden. Peyton explained that treatment with hydrogel-formulated intravesical mitomycin did not result in sustained decrements in functioning and symptom burden. Previous work identified the MCID,12 the difference in scores that would be considered clinically meaningful. Change in scores from baseline can be classified as either ‘improving’ or ‘worsening’ QoL using the MCID. At 3- and 12-months post-treatment, mean PRO scores did not surpass the ‘worsening’ MCID thresholds for any outcomes, as illustrated in Figure 1, indicating that treatment with intravesical mitomycin did not negatively impact QoL.
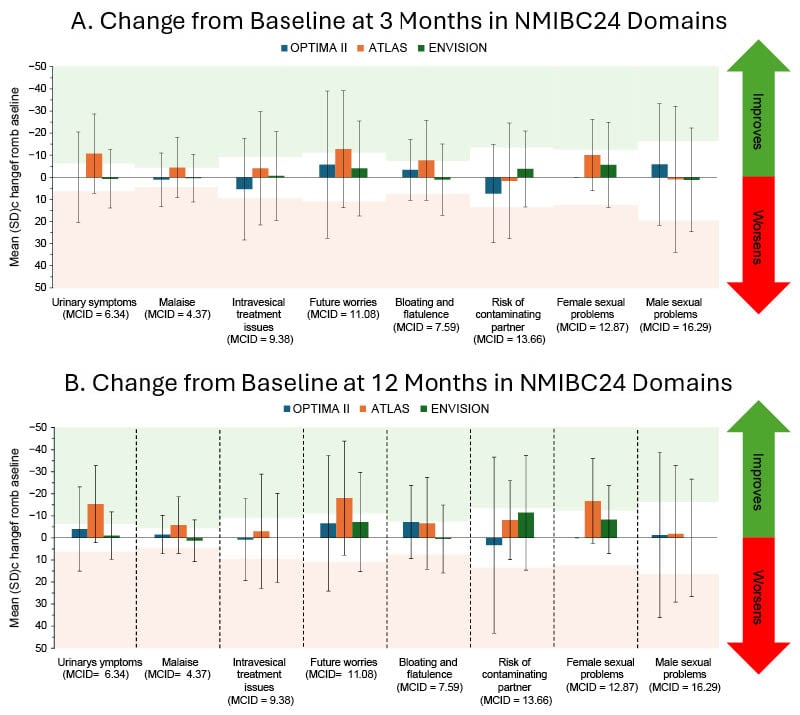
Figure 1: Change from baseline in patient-reported outcomes following intravesical mitomycin treatment.
Patients involved in three clinical trials, OPTIMA II (n=63), ENVISION (n=240), and ATLAS (n=142), who had received six once-weekly instillations of intravesical mitomycin were evaluated using the EORTC-QLQ-NMIBC24 to inform patient reported symptom burden at baseline, 3 months, and 12 months after treatment. Outcomes after 3 (Figure 1A) and 12 (Figure 1B) months are presented as mean ± standard deviation. Previous work identified the MCID for each outcome,12 with green and red shaded areas to indicate the MCID threshold for improvement and worsening, respectively, per outcome. Mean PROs did not surpass the ‘worsening’ MCID thresholds for any outcomes.
Figure adapted from Peyton et al.16 2025
MCID: minimal clinically important difference; NMIBC: non-muscle-invasive bladder cancer; SD: standard deviation.
Peyton described meaningful improvements in urinary symptoms, malaise, and future worries at both 3- and 12-month visits in the ATLAS trial. At 3 months, meaningful improvements in bloating and flatulence were also observed, whereas at 12 months, improvements to female sexual problems were observed.13 Peyton described that intravesical mitomycin provides a potential non-surgical treatment option that avoids repeat invasive procedures and maintains, and in some cases improves, QoL for patients with LG-IR-NMIBC.
Hydrogel-Based Chemotherapy for Urothelial Carcinomas
The ATLAS13 and ENVISION14 trials were based on the treatment of LG-IR-NMIBC with a reverse thermal hydrogel containing mitomycin, illustrated in Figure 2. Such non-surgical treatment alternatives for urological cancers aim to combat many of the adverse effects of surgical treatments, including physiological side effects and patient-reported QoL factors, while also improving treatment durability.
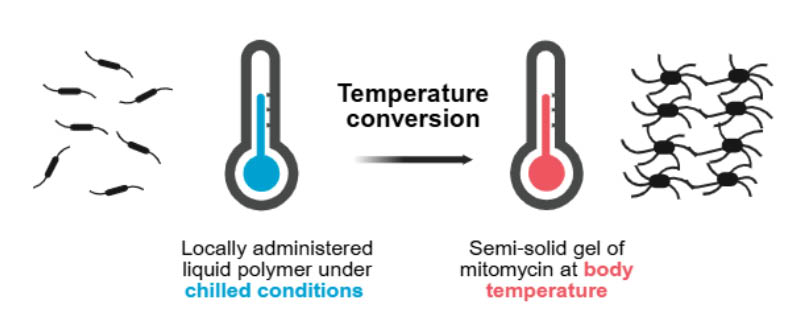
Figure 2: Reverse thermal hydrogel.
The gel has reverse thermal properties: when it is administered as a cooled solution, it is viscous but fluid enough to conform to the urinary tract anatomy. As the gel reaches body temperature, its viscosity increases to become a semi-solid gel which dwells in the ureter and renal pelvis, providing a sustained exposure of mitomycin.20
Figure is based on information from Urogen20 and created with BioRender.com.
A hydrogel-based chemotherapy with mitomycin was approved by the FDA in April 2020 for the treatment of patients with LG-UTUC.17, 18 This approval was based on data from the OLYMPUS trial, where mitomycin for pyelocaliceal solution (4 mg/mL) was used as a primary treatment for LG-UTUC, resulting in clinically significant disease eradication.19 Pyelocaliceal solution refers to the administration into the renal pelvis and calyces of the kidney. It is approved for retrograde administration via a ureteral catheter or antegrade administration through a nephrostomy tube.17 The delivery system and reverse thermal properties (Figure 2) of the gel ensure that the mitomycin solution coats the upper urinary tract.
The OLYMPUS Trial
The OLYMPUS trial19 was an open-label, single-arm, Phase III trial that recruited patients from 24 academic sites across the USA and Israel. In total, 71 patients with new or recurrent LG-UTUC were treated with six once-weekly instillations of mitomycin for pyelocaliceal solution (4 mg/mL) via retrograde catheter. Of these patients, 61 received all six instillations. The primary outcome was CR, defined as a negative 3-month ureteroscopic evaluation, negative cytology, and negative for-cause biopsy. Of the enrolled patients, 58% (n=41) had a CR at the primary endpoint evaluation (around 3 months). Of the patients with a CR, the median follow-up was 11.0 months (interquartile range [IQR]: 5.1–12.4). The most frequently reported all-cause adverse events were ureteric stenosis in 44% (n=31), UTI in 32% (n=23), haematuria in 31% (n=22), flank pain in 30% (n=21), and nausea in 24% (n=17) of 71 patients. Overall, 25% (n=19) of 71 patients had study drug-related or procedure-related serious adverse events (SAE). No deaths were regarded as related to treatment.
Long-Term Durability of Hydrogel Mitomycin for Upper Tract Urothelial Carcinoma
At AUA 2025, Brian Hu, Associate Professor of Urology at Loma Linda University, California, USA, presented a long-term follow-up (LTFU) from patients included in the OLYMPUS trial.21,22 The 41 patients who achieved a CR during the trial, were followed up for 12 months after treatment with optional monthly maintenance doses of mitomycin in the same hydrogel formulation. At 12 months, 23 patients remained in CR and, at 15 months, 20 patients remained at CR and entered the LTFU study. The LTFU was a non-interventional study where supervising physicians provided semi-annual updates on each patient’s disease status for 5 years until disease recurrence, progression or death.
For all patients who achieved CR in the OLYMPUS trial (n=41), the median follow-up was 28.1 months (95% CI: 13.1–57.5), with a Kaplan–Meier estimated duration of response (DoR) of 47.8 months (95% CI: 13.0–not estimable [NE]). Kaplan–Meier survival analysis is illustrated in Figure 3. In patients who entered the LTFU study (n=20), the median follow-up was 53.5 (95% CI: 27.9–65.3) months, with a DoR NE due to the low event rate (95% CI: 43.5–NE). No difference was reported between patients with new onset UTUC at baseline compared to those who had experienced recurrences before treatment. Of the patients included in the LTFU, 75% had no evidence of recurrence at the last follow-up, 10% of patients (n=2) experienced UTUC tumour recurrence, and 15% (n=3) of patients died, reported as unrelated to study treatment. These data illustrate a clinically meaningful long-term response in patients treated with hydrogel-formulated mitomycin, which lasted on average nearly 4 years (47.8 months–3.9 years), irrespective of whether the patient enrolled into the OLYMPUS clinical trial with multiple previous recurrences.
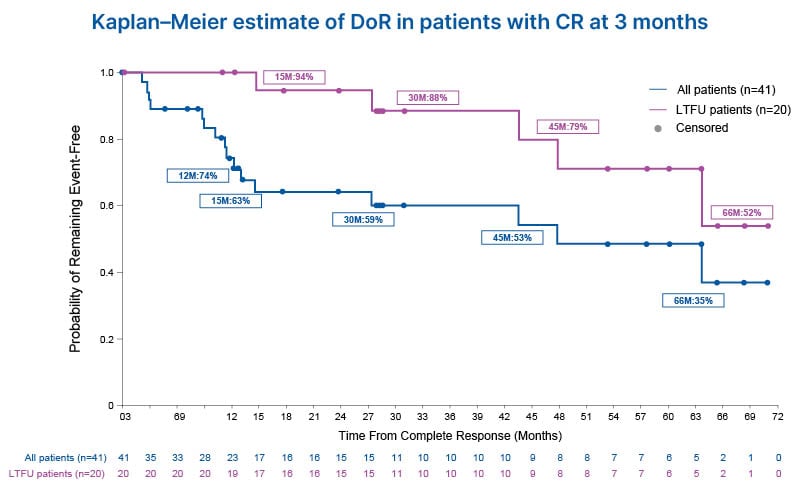
Figure 3: Kaplan–Meier estimate of duration of response in patients from the OLYMPUS trial.
Patients with confirmed LG-UTUC (n=71) were treated with six once-weekly instillations of hydrogel-formulated mitomycin (4 mg/mL) for pyelocaliceal administration. Patients who remained in CR (negative 3-month ureteroscopic evaluation, negative cytology, and negative for-cause biopsy) after 12 months were included in the LTFU study (n=20). Kaplan–Meier survival analysis was conducted, with an estimated DoR of 47.8 months (95% CI: 13.0–NE) for all patients who received CR in the OLYMPUS trial.
Figure adapted from Hu et al.21
CR: complete response; DoR: duration of response; LG-UTUC: low-grade upper tract urothelial cancer; LTFU: long-term follow-up; M: months.
Hu explained that, although the study was a post hoc analysis,21 the data presented give insight into the long-term durability of hydrogel formulation chemotherapies for UTUC. Hu then described an ongoing uTRACT registry23 that will build on the insights from the LTFU study by providing an opportunity to collect and evaluate real-world data in a larger sample, to further inform the use of mitomycin for pyelocaliceal solution in patient with UTUC.
Hydrogel-Based Chemotherapy for Non-muscle Invasive Bladder Cancer: Ongoing Clinical Research
Following the FDA approval of a reverse thermal hydrogel formulation of mitomycin for UTUC, ongoing trials are evaluating the safety and efficacy of an intravesical formulation of mitomycin in reverse thermal hydrogel as a non-surgical treatment option for LG-IR-NMIBC. At AUA 2025, data were presented from a LTFU of the OPTIMA II Phase II study24 and from the ENVISION Phase III study,25 both assessing the intravesical administration of mitomycin-containing reverse thermal hydrogel as a treatment for LG-IR-NMIBC.
Long-Term Durability of Hydrogel Mitomycin for Non-muscle Invasive Bladder Cancer
Neal Shore, Medical Director of the Carolina Urologic Research Center and Doctor of Urology at the Atlantic Urology Clinics, Myrtle Beach, South Carolina, USA, presented results from LTFU of the Optima II trial.24 Twenty-five patients remained in CR at 12 months and 17 of these patients were included in the LTFU study. This non-interventional study followed patients over 4 years or until disease recurrence, progression, or death. Disease status was reported by physicians, who gave semi-annual updates on each patient. In the OPTIMA II study, most patients had recurrent disease at baseline (77.8%) and had undergone multiple TURBT procedures prior to enrolment. Following intravesical mitomycin treatment, the median Kaplan–Meier estimate of DoR was 24.2 months (95% CI: 9.72–47.18), with a median follow-up time of 33.6 months (95% CI: 10.78–42.94). Overall, 53.7% (n=22) of patients experienced recurrence, with the majority of these patients (n=20) having low-grade disease. One patient progressed to high-grade disease and one patient died due to a cardiac disorder. At the end of the LTFU, five patients remained disease free. Shore described how these results demonstrate a clinically meaningful long-term response from intravesical mitomycin treatment, and that this treatment may represent a durable and well-tolerated non-surgical alternative to TURBT for patients with LG-IR-NMIBC.
Following the successful completion of the Optima II trial, the ENVISION trial14,26 is now ongoing. It is a single-arm, open-label, Phase III trial to investigate the safety and efficacy of intravesical mitomycin as treatment for patients with LG-IR-NMIBC.
The ENVISION Trial Results and Long-Term Follow-Up
Following screening and enrolment, 240 patients received six once-weekly intravesical instillations of hydrogel formulation mitomycin (1.33 mg/mL). All patients received at least one of the six planned doses in an outpatient setting and 228/240 patients received all six doses. All patients were assessed for the primary endpoint: CR at around 3 months after the first instillation. CR was defined as negative white light cystoscopy, negative urine cytology and, when required, a negative for-cause biopsy. Patients with a CR at 3 months entered the LTFU period of the study. Patients with a non-CR underwent investigator-designated standard of care treatment of remaining lesions, then entered the LTFU. The LTFU portion of the study is expected to run until 2028, with patients monitored every 3 months until recurrence, progression, death, or end of study at 63 months. Upon first recurrence or progression, patients are offered investigator-designated standard of care treatment of lesions, followed by an end of study evaluation 3 months later.
At AUA 2025, Sandip Prasad, Doctor of Urology at Morristown Medical Center, Atlantic Health System, Garden State Urology, Morristown, New Jersey, USA, presented ongoing results from the primary endpoint evaluation of the ENVISION study.24,26 At 3 months, a high proportion (79.6%) of patients had a CR. To inform potential treatment durability, the 3-month CR dataset was used to estimate DoR using Kaplan Meier survival analysis (Figure 4). For patients who achieved CR at 3 months, the probability of remaining event-free 18 months later was estimated at 80.6%.
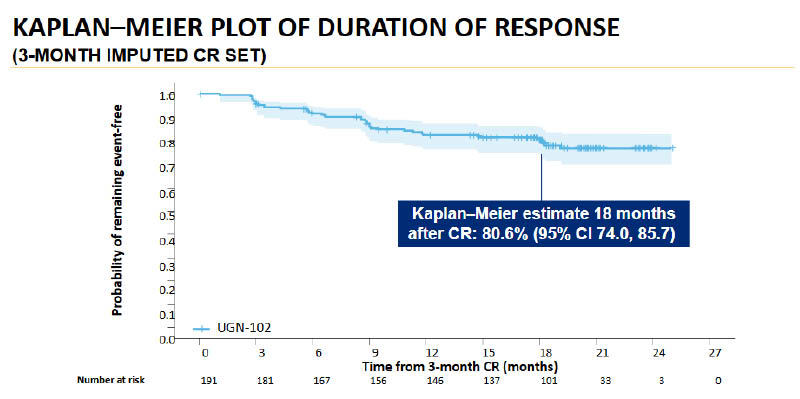
Figure 4: Kaplan-Meier estimate of duration of response in patients from the OLYMPUS trial.
Patients with confirmed LG-IR-NMIBC (n=240) were treated with six weekly instillations of hydrogel-formulated mitomycin (1.33 mg/mL) for intravesical administration. Patients who remained in CR (negative white light cystoscopy, negative urine cytology and, when required, a negative for-cause biopsy) after 3 months were included in the LTFU study (ongoing). Kaplan-Meier survival analysis was conducted on 3-month CR data.24
Figure adapted from Prasad et al.24
LG-IR-NMIBC: low-grade intermediate-risk non-muscle invasive bladder cancer; LTFU: long-term follow up;
CR: complete response.
Prasad described the treatment as well-tolerated and provided insight into the side effects reported at the 3-month follow up. Treatment-emergent adverse events (TEAE) were reported as follows: 22.5% (n=54) of patients with dysuria, 8.3% (n=20) with haematuria, 7.1% (n=17) with UTI, 6.7% (n=16) with frequent urination, and 5.4% (n=13) of patients reported fatigue. Two instances of urinary retention and atrial fibrillation were reported. Single instances of metastatic lung cancer, cerebrovascular accident, and pancreatic adenocarcinoma were reported; however, all were unrelated to treatment as per principal investigator judgment. Three patients died from pneumonia, cardiac failure, or unknown reason, all of which were assessed as unrelated to treatment. Two treatment-relate SAEs were reported: urethral stenosis in one patient (0.4%) and urinary retention in one patient (0.4%), both of which resolved.
Prasad described the CR, durability of response, and benefit-risk profile of intravesical hydrogel-formulated mitomycin as favourable, supporting its further investigation as a non-surgical alternative for LG-IR-NMIBC patients.
Hydrogel-Based Immunotherapy for Non-muscle Invasive Bladder Cancer: Emerging Insights
Immunotherapy is a well-established treatment option for NMIBC. The Bacille Calmette-Guérin (BCG) vaccine is used routinely for preventing or delaying tumour recurrence following high-grade NMIBC resection.4 BCG is administered intravesically to trigger a local immune response within the bladder, activating immune cells including macrophages and T cells to target and destroy cancer cells. Although most patients have an initial response, over 50% of initial responders will experience recurrence or progression,4 illustrating the need for new, more durable treatment options.
Phase I Study: Zalifrelimab for NMIBC
At AUA 2025, Jay Raman, Professor and Chair in the Department of Urology and Department of Surgery at Penn State Cancer Institute, Hershey, Pennsylvania, USA, presented the results from a Phase I, dose-escalation study investigating hydrogel formulated zalifrelimab for the treatment of recurrent NMIBC.27 Zalifrelimab is a monoclonal antibody against cytotoxic T-lymphocyte antigen-4 (CTLA-4) which functions as an immune checkpoint inhibitor, by neutralising the inhibitory effect of CTLA-4 on T cell activation.27
The data reported at AUA 2025 were part of an ongoing, multi-component clinical trial with up to 30 patients included per arm.27 Arm A, reported by Raman at AUA 2025, investigated the safety, efficacy, and pharmacokinetics of intravesically administered, hydrogel-formulated zalifrelimab monotherapy as a dose escalation study.
Arms B and C are currently ongoing (as of April 2025)28 and will investigate a dose escalation of hydrogel formulated zalifrelimab in combination with imiquimod (Arm B), and gemcitabine (Arm C), respectively.28 Imiquimod, a toll-like receptor 7 agonist with immune-stimulating anti-tumour effects, and gemcitabine, a chemotherapy agent, will be administered intravesically in a non-hydrogel formulation prior to the instillation of reverse thermal hydrogel-formulated zalifrelimab.28,29
In Arm A, key inclusion criteria included patients with recurrent NMIBC with low-grade disease or high-grade disease (Stages Ta or T1) and/or carcinoma in situ (CIS), who had failed at least one prior course of intravesical therapy. For enrolment, patients were required to have had resection of papillary tumours and obvious areas of CIS fulgurated during screening or within 6 weeks prior to screening.
During the study, patients received six weekly intravesical instillations of hydrogel-formulated zalifrelimab across four cohorts: 100 mg (n=3), 300 mg (n=6), 500 mg (n=8), and 700 mg (n=3). A total of 20 patients received at least one dose, and 19 patients received all six doses. Raman reported a favourable safety profile from trial Arm A. The reported TEAEs were mild or moderate in severity except for one severe event, a Grade 3 UTI which was considered related to the procedure, but not the treatment, and did not result in treatment discontinuation. The most common TEAEs were dysuria, haematuria, urinary retention, UTI, headache, and nausea. Raman commented that no dose-dependency of TEAEs or dose-limiting toxicities were observed.
Patients that received at least one treatment dose, and completed the efficacy assessment, were evaluated in this study. Of the evaluated patients, 46.2% (n=6/13) with Ta/T1 disease and 33% (n=3/6) with CIS +/- Ta/T1, respectively, were recurrence free or had a CR at 3 months after initial instillation. During follow-up, one patient in the 100 mg cohort with high-grade Ta disease remained recurrence-free at 9 months; 75% (n=3/5) patients in the 300 mg cohort with Ta/T1 disease remained recurrence-free at 15 months; and 25% (n=1/4) of patients in the 500 mg cohort with Ta/T1 disease remained recurrence-free at 6 months.
Across all dose cohorts, the median duration of detectable zalifrelimab in urine was ≥9.7 hours at Week 1 and Week 6. The maximum concentration in urine (Cmax) plateaued at 500 mg. Systemic exposure was only detected in one patient who was part of the 700 mg cohort. In this patient, exposure was 50-fold lower than that achieved with systemic administration and was 2,550-fold lower than the urine Cmax. Raman concluded that the intravesical delivery of hydrogel-formulated zalifrelimab allowed sustained exposure of the drug to the target organ, the bladder, while limiting systemic exposure, thus mitigating the risk of systemic immune-related toxicities associated with CTLA-4 inhibition.27
Conclusion and Outlook
Surgical treatment for urological cancers often lacks durability, requiring multiple procedures due to disease recurrence or progression. These data presented at AUA 2025 highlight the potential of reverse thermal gel formulations of anti-cancer agents, including mitomycin as an effective alternative to repetitive surgeries for treating different forms of low-grade urothelial cancers, and zalifrelimab for the treatment of recurrent NMIBC. Future work is ongoing to further evaluate the safety and efficacy of these investigational therapies, with the hope of providing an alternative to surgery for patients with NMIBC, as has been demonstrated with LG-UTUC.