Meeting Summary
This symposium was held at the 30th Annual International Congress of the World Muscle Society (WMS) 2025, in Vienna, Austria. Speakers raised awareness of the importance of natural history studies to support the development of novel treatments in Becker muscular dystrophy (Becker), and presented the latest clinical data for the investigational agent, sevasemten.
Becker is a rare, serious muscular dystrophy that presents predominately in males. It is caused by a mutation in the dystrophin gene, which leads to contraction-induced muscle injury and progressive impairment of mobility and cardiac function, amongst other effects. There are currently no approved treatments for Becker.
Erik Niks, Consultant and Paediatric Neurologist at Leiden University Medical Center (LUMC), the Netherlands, explained how natural history studies provide better understanding of disease severity and progression in Becker. Using the North Star Ambulatory Assessment (NSAA) as a clinically meaningful longitudinal measure of function, Niks described the development and validation of a new Becker NSAA prediction model, and its importance in providing benchmarks for treatment outcomes in Becker clinical studies. Craig McDonald, Director of the Muscular Dystrophy Association (MDA) Neuromuscular Diseases Clinic, University of California, Davis, USA, shared updates from the sevasemten Becker clinical programme, including recently released data from the adolescent population in the CANYON study. Sevasemten treatment was associated with significant decreases in biomarkers of muscle damage. In addition, a Becker prognostic model illustrated long-term stabilisation of disease progression across 18 months (versus predicted natural history control) in sevasemten-treated participants continuing from CANYON into the MESA extension study.
Introduction
Becker is a serious, progressive neuromuscular condition that is X-linked, affecting approximately one in 18,000 male births.1,2 It is caused by mutations in the DMD gene that encodes dystrophin and is driven by contraction-induced muscle damage.1,3,4 Characteristic clinical features include progressive muscle weakness and cardiomyopathy, although multiple body systems may be affected.1 Once disease progression begins, individuals living with Becker are on an irreversible path to loss of mobility, function, and independence.5,6 There is currently no approved treatment for Becker, and disease management mainly consists of symptomatic treatment to preserve cardiac function, and exercise and physiotherapy to help retain mobility.1,3 Harnessing new insights from natural history studies, the speakers described the latest clinical progress in the development of sevasemten, a novel agent for the treatment of Becker.
Becker Natural History and Modelling
Natural History of Becker
Introducing the topic of natural history, Niks began: “Becker muscular dystrophy natural history data have evolved hugely over recent years, helping us to characterise disease progression, and identify sub-populations in decline.” Within these studies, the validated NSAA has been used as a longitudinal measure of ambulatory function in Becker.5-9 The NSAA consists of 17 items to test different aspects of motor function (e.g., rise from chair, walk, stand, jump), scored as 0 (cannot perform), 1 (can perform with compensation due to muscle weakness), or 2 (can perform normally), giving a total maximum score of 34 points.6 As Niks illustrated in several patient videos, the NSAA test items clearly correspond to activities in daily life; for example, the item ‘rise from chair’ may reflect an individual’s level of independence in toileting or getting out of bed, while the items ‘jump/hop/run’ and ‘walk’ link with everyday mobility and participation in recreational activities. Thus, NSAA scores are clinically meaningful in a real-world context.
Combined data from Becker natural history studies show consistent mean annual rates of decline in NSAA between 1.0–1.7 points.5-9 Most recently, two long-term (3- and 6-year follow-up) studies demonstrated that this rate of decline is linear, and also most evident, in patients with baseline NSAA values of 10–32.5,9 The studies also revealed that the rate of decline differs across genotypes, with the greatest decline consistently associated with the common del 45–47 genotype.5,9 Thus, overall, natural history data have shown that functional decline in Becker, as measured by NSAA, is predictable, which has led to the development of a prediction model.
Prediction Modelling
Niks explained that prediction models for natural history disease trajectories can provide contextualisation for clinical trial outcomes, which is especially valuable over multi-year periods in Becker and other muscular dystrophies for which placebo-controlled studies are not feasible. A predictive model to examine NSAA trajectories and changes in individuals living with Becker was developed based on longitudinal natural history data from the Netherlands.10 The input data set was the changes from baseline NSAA total score from the Dutch cohort: an ambulatory sub-population (n=24) with a median follow-up of 37.2 months, and a baseline NSAA total score of 23.9. Several baseline predictors were applied: NSAA, age, 4-stair climb velocity, 10-metre walk/run velocity, and time to rise from floor, and the proportion of variability in the model explained by these predictors was 0.53.10
The model was then validated against two independent published studies of natural history data by Bello et al.6 (2016, Italy; n=69) and De Wel et al.8 (2024, Belgium; n=21), which both showed a significant decline in NSAA score from baseline to follow-up.6,8 The prediction model performed well against these study data, with predicted means closely matching observed values.10 Compared to Bello et al.,6 the predicted mean change in NSAA at 12 months (-0.9; 95% CI: -1.3–-0.5) closely matched the observed value (-0.9). Compared to De Wel et al.,8 the predicted mean change in NSAA showed smaller declines of -0.9 (95% CI: -1.2–-0.7) versus -1.3 at 9 months, and -1.9 (95% CI: -2.4–-1.4) versus -2.5 at 18 months, although the absolute differences were within 1 unit.
Niks concluded: “The importance of studying natural history cannot be emphasised enough, not only in Becker but in many neurological diseases. It’s central to the understanding of both disease progression and severity, but also for work on clinical endpoints. The prediction models for these trajectories can also provide important benchmarks for the outcome of patients receiving novel therapies (as discussed in the following presentation), and we think this is especially valuable for chronic, slow, progressive diseases like Becker muscular dystrophy.”
Advancements with an Investigational Agent: Sevasemten Clinical Programme Update
Contraction-induced muscle damage is the root driver of disease progression in muscular dystrophy. In Becker, fast (Type II) skeletal muscle fibres become disproportionately injured by contraction due to the lack of a fully functional dystrophin protein.11-13 McDonald explained that sevasemten, a first-in-class, oral, fast myofibre (Type II) myosin inhibitor, has been designed to prevent contraction-induced muscle damage while preserving strength:14 “It allows muscles to function normally, but protects them from higher loads and the risk of contraction-induced injury.” This action is independent of the specific dystrophin mutation (‘mutation agnostic’) and, due to being inactive against slow and cardiac myofibre (Type I) myosin, sevasemten does not directly affect cardiac function.14 The sevasemten clinical trial programme in Becker is ongoing, using both biomarkers of muscle damage and functional assessments to evaluate the treatment’s ability to protect susceptible muscle fibres and impact disease progression. McDonald summarised findings from the clinical programme, including recently released adolescent data from the CANYON study and the latest long-term data from the MESA extension study. Interpretation of the study findings was enhanced by the availability of the Becker NSAA prediction model, as defined by Niks in the preceding presentation.
ARCH and DUNE
ARCH was a Phase I, open-label, dose-finding, single-centre study that assessed the safety and pharmacokinetics of sevasemten (10–20 mg daily) in 12 adult males (18–55 years) with Becker and moderately severe functional impairment (median NSAA: 15.5).15,16 Based on the full 24 months of study data, sevasemten was well tolerated, with sevasemten 10 mg/day selected as the optimal clinical dose. In terms of efficacy, early and rapid reductions in biomarkers of muscle damage (creatine kinase [CK] and fast skeletal muscle troponin I, Type 2 [TNNI2])17 were observed, sustained to 24 months.15 There was also stabilisation of function, with a mean change in NSAA score of -0.2 points at 24 months, notably diverging from the average natural history change of -2.4 points.
DUNE was a Phase II, placebo-controlled exercise-challenge study that examined the effect of sevasemten on the transient elevation in circulating muscle injury proteins, TNNI2 and CK, following exercise.18 Including nine adults with Becker, the study showed that sevasemten-treated participants had a significant reduction in injury biomarkers during a period of normal activity (compared to placebo), as well as a significant reduction in post-exercise increases in CK. Sevasemten was also well tolerated across 16 weeks in this study.
CANYON
The Phase II CANYON study was a 12-month, multicentre, randomised, double-blind, placebo-controlled trial that evaluated the safety and pharmacokinetics of sevasemten, and also included muscle biomarkers and function (NSAA) as key endpoints.19,20 CANYON enrolled ambulatory males aged 12–50 years with a dystrophin mutation, a Becker phenotype, and, based on the key learnings from natural history studies (discussed earlier), adults were required to have an NSAA of 5–32 points, enriching the study population for those participants most likely to show linear progression over 12 months. A total of 40 adults and 29 adolescents were enrolled and randomised 2:1 to receive oral sevasemten or placebo: adults 10 mg daily (n=28) or placebo (n=12); adolescents 5 or 12.5 mg daily (n=20) or placebo (n=9). The primary efficacy endpoint was change from baseline in CK averaged across Months 6, 9, and 12; the key secondary endpoint was change from baseline to Month 12 in NSAA score. McDonald highlighted the statistically significant imbalance in baseline levels of functional severity in the adult population, with participants in the sevasemten group being more severely affected than placebo (mean NSAA: 18.4 versus 24.2 points, respectively), while the adolescent participants were relatively mildly affected overall (mean NSAA: 31.3 versus 29.3 points, respectively).
The study met its primary efficacy endpoint in adults, with a statistically significant reduction from baseline in CK of 28% with sevasemten versus placebo (least squares [LS] mean between-group difference; p=0.02) sustained across Months 6–12. There was also a rapid and sustained decrease in plasma TNNI2 of 77% (LS mean between-group difference; p<0.001), which McDonald described as “impressive and encouraging.” In the adolescent population, participants treated with sevasemten 12.5 mg/day showed reductions from baseline in biomarker levels similar to those seen in adults, with a 41% decrease in CK, and an 84% decrease in TNNI2, versus placebo (both LS mean between-group differences; p<0.05; Figure 1). McDonald noted that the 12.5 mg/day dose was “pharmacokinetically similar” to the 10 mg/day dose in adults, and highlighted the efficacy of sevasemten in both adult participants and the more mildly affected adolescent population.
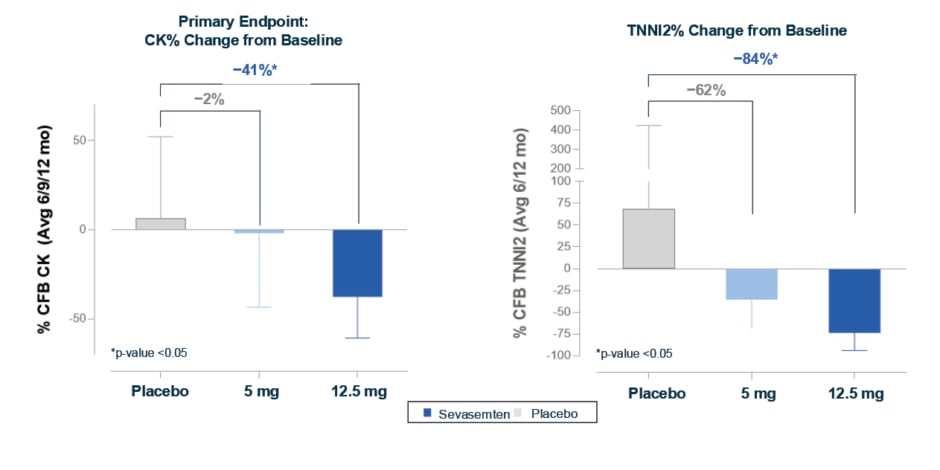
Figure 1: Adolescents treated with sevasemten 12.5 mg had significant decreases in creatine kinase and fast skeletal troponin, biomarkers of muscle damage, similar to changes in adults (CANYON study).19
*p-value <0.05.
LS means, LS mean differences, and 95% CI shown for the safety population; CK and TNNI2 values were log-transformed. LS means, LS mean differences, and CIs were back-transformed to percent scale.
Avg: average; CFB: change from baseline; CK: creatine kinase; LS: least squares; mo: months; TNNI2: fast skeletal troponin.
In addition, adults treated with sevasemten showed functional stabilisation (NSAA, key secondary endpoint), while the placebo group declined in line with natural history. Although the study was not powered to detect a difference versus placebo, change in NSAA over 12 months in sevasemten-treated participants equated to a difference of 1.12 points greater than participants treated with placebo, (p=0.16 versus placebo). In participants treated with sevasemten, 63% were considered NSAA responders (stable or improved score after 12 months; odds ratio: 3.4; p=0.16). There were also trends in favour of sevasemten across other secondary endpoints (4-stair climb; 100 m timed test; 10 m walk/run) at 12 months, adding further support to the functional effects of sevasemten in this double-blind placebo-controlled study.
In terms of safety, sevasemten was well tolerated at all doses in both adults and adolescents across 12 months of treatment in CANYON, with no safety concerns identified. The most common adverse events were headache and dizziness, which were generally transient effects. Due to its specificity for skeletal muscle over cardiac muscle, sevasemten is not expected to produce adverse cardiac effects, but the absence of any toxicity on the myocardium in the CANYON study (which included participants with cardiomyopathy) was described as ‘reassuring’.
GRAND CANYON
Following the observations of NSAA stabilisation, the 12-month CANYON study was expanded to include an additional cohort of adult participants referred to as GRAND CANYON. The primary endpoint of this pivotal cohort study is NSAA at 18 months, powered at >90% to demonstrate a statistically significant difference between sevasemten and placebo groups, as supported by data from the CANYON study. Currently ongoing, the GRAND CANYON study has enrolled 175 participants (NSAA 5–32) to evaluate the effect of sevasemten on biomarkers of muscle damage and functional measures versus placebo over 18 months.20 Topline results are expected in late 2026.
MESA: Long-Term Data
MESA, an open-label extension study, was planned to assess the long-term (3-year) safety and durability of sevasemten treatment effects in Becker.21 MESA is enrolling participants from the ARCH, DUNE, CANYON, and GRAND CANYON studies and, to date, 99% of eligible participants have enrolled (n=85). On the high level of enrolment, McDonald commented: “I think it’s indicative of how well these patients are feeling, and their motivation, that 99% of eligible patients have chosen to carry over and enrol in the MESA open-label extension trial. Sevasemten is a well-tolerated small molecule, and we’ve seen very little dropout in the development programme.” Initial output from the MESA study was discussed in the context of natural history data, as well as versus the Becker prognostic model. On entering MESA, all participants received sevasemten (placebo participants were transitioned) but remained blinded to their original treatment assignment. After 6 months in MESA, former CANYON participants who had transitioned from placebo to sevasemten (n=12) showed stabilisation of disease progression, with a change in NSAA score of 0.2 points (Figure 2). Moreover, participants who remained on sevasemten across both studies (n=28) had an increase in NSAA of 0.8 points versus original baseline after a total of 18 months of treatment (Figure 2). Applying baseline characteristics from the treated patient group in CANYON, the Becker prognostic model developed by Niks predicted an NSAA trajectory of -2.2 points over the 18-month time period, equating to an overall improvement of 3.0 points in the sevasemten group, with 92% of participants improved versus their predicted NSAA score.
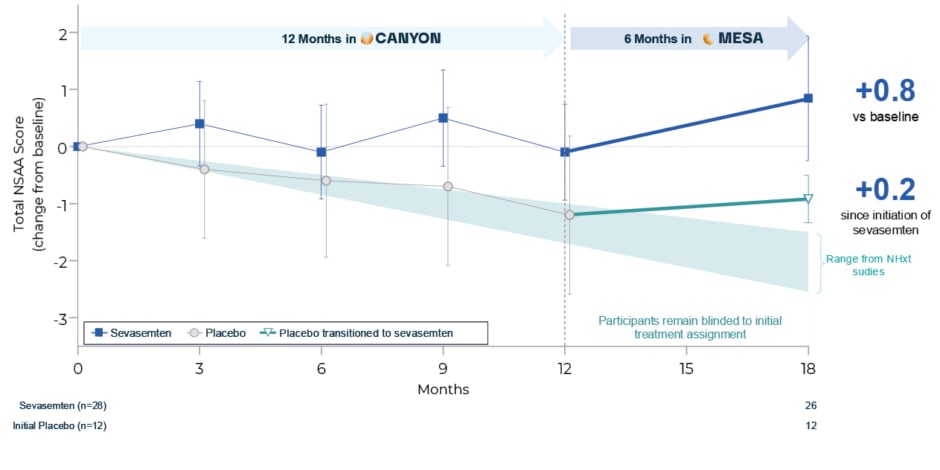
Figure 2: After transitioning to MESA, CANYON participants showed an increase in North Star Ambulatory Assessment after 18 months.
Through Month 12, LS means and 95% CI shown for safety population; post-Month 12 timepoints, observed means and 95% CI are presented.
LS: least squares; NSAA: North Star Ambulatory Assessment; vs: versus.
After 3 years of sevasemten treatment (12 months in MESA), former ARCH participants (n=12) displayed what McDonald described as “long-term stabilisation of disease progression” with a change from original ARCH baseline of 0.2 points versus a predicted decline of -4.4 points in NSAA (difference of 4.6 points; Figure 3). In total, 89% of ARCH participants achieved higher NSAA scores versus those predicted by the Becker prognostic model.
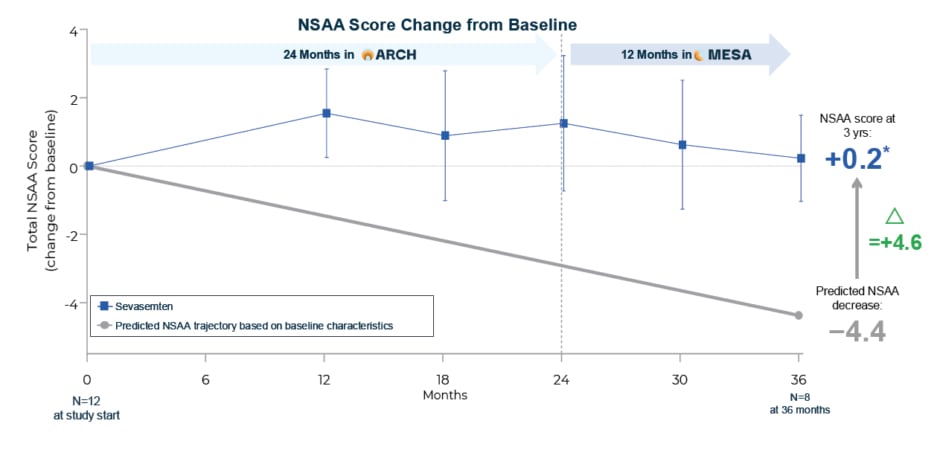
Figure 3: After transitioning to MESA, ARCH participants remained stable versus a predicted −4.4 point NSAA decline after 3 years.
*Subject with meniscal tear at Month 15 excluded from subsequent NSAA measures.
Means and 95% CI shown for safety population; natural history comparators not available for timepoints earlier than 12 months.
NSAA: North Star Ambulatory Assessment; yrs: years.
McDonald summarised that sevasemten continues to be investigated in Becker in the ongoing MESA and GRAND CANYON studies,20,21 as well as in Duchenne muscular dystrophy in the Phase II LYNX and FOX studies,22,23 and concluded: “We have a rigorous clinical development programme across both Becker and Duchenne muscular dystrophy, which is providing a lot of exciting data in terms of the long-term implications of this therapy.”







