Abstract
Introduction: Acute myeloid leukaemia (AML) is a rapidly progressing myeloid neoplasm caused by the clonal expansion of primitive haematopoietic stem cells (blasts) in the bone marrow, leading to bone marrow failure and systemic symptoms. It is a rare condition, with approximately 2,900–3,100 annual cases in the UK. AML is a highly genetically and clonally heterogeneous condition. FLT3 mutations, including internal tandem duplication and tyrosine kinase domain variants, occur in 30% of cases and influence prognosis and treatment. Advances in genetic diagnoses have led to targeted therapies, emphasising the importance of early mutation detection to optimise treatment and improve outcomes. However, variability in diagnostic approaches across UK laboratories presents challenges in achieving uniform care. This Delphi study aimed to support the optimal care of FLT3 mutated AML in the UK.
Methods: A modified Delphi methodology consisting of two rounds was employed in this study. A literature review on the topic of AML was performed to identify the problem. A steering group of 10 experts from the UK attended a virtual meeting in July 2024. During this meeting, and guided by an independent facilitator, the group identified four main domains: A) defining the optimal role and timing of testing for patients with AML, including FLT3 status testing and retesting at relapse or determination of refractory disease; B) determining which patients are unfit/unsuitable for intensive chemotherapy; C) defining and treating patients who are FLT3+, unfit for chemotherapy, and refractory or relapse; D) defining the position of gilteritinib as a treatment option for patients with FLT3+ mutated AML. From discussions of these domains, 31 statements were agreed upon and developed into an online survey for testing with a wider panel of healthcare professionals in the UK. Stopping criteria for consensus rounds were defined as a 1-month period to collect responses, a target of 100 responses, and 90% of statements passing the threshold for consensus (≥75% agreement).
Results: In Round 2, a total of 100 responses were received from haematologists with experience in the management of AML in the UK. Overall, 65% of statements achieved ≥90% agreement (n=20/31), an additional 29% achieved ≥75–90% agreement (n=9/31), and 6% of statements did not achieve ≥75% agreement (n=2/31). As the predetermined stopping criteria were met with 94% of statements achieving consensus, no further rounds of survey were conducted.
Conclusion: Based on the agreement levels achieved, the steering group formulated a set of recommendations to support the optimal care of patients with AML in the UK. It is expected that the proposed pathway will standardise the diagnostic and treatment approach, as well as improve patient outcomes.
INTRODUCTION
Acute myeloid leukaemia (AML) is a rapidly progressing myeloid neoplasm. The main pathogenetic mechanism of the disease is the clonal expansion of primitive haematopoietic stem cells, generating large numbers of blasts with differentiation block, in the bone marrow.1 This results in ineffective erythropoiesis and megakaryopoiesis leading to bone marrow failure and signs and symptoms affecting different organs and systems.1
Despite the fact that AML is considered a rare condition, approximately 2,900–3,100 people are diagnosed with it each year in the UK.2,3 The incidence of the disease increases in patients over 75 years old. Remission rates are related inversely to age, with rates exceeding 65% being achieved among patients younger than 60 years.3,4
The primary cause of AML development is the acquisition of somatic mutations in haematopoietic stem and progenitor cells, resulting in clonal haematopoiesis. Some mutations, such as DNMT3A, TET2, and ASXL1, occur during clonal haematopoiesis, while others, such as FLT3, NRAS, and RUNX1, are acquired dynamically during the course of the disease.5 In some individuals, AML develops de novo without prior clonal haematopoiesis.
Mutations in the FLT3 gene, including internal tandem duplications (FLT3-ITD) and tyrosine kinase domain (FLT3-TKD) point mutations, are among the most common molecular aberrations in AML, occurring in approximately 30% of newly diagnosed cases, and are associated with poorer prognosis, higher relapse rates, and shorter overall survival (OS). FLT3-ITD mutations are the most common type, seen in approximately 25% of all AML cases. The prognostic significance of FLT3-TKD mutations, which are present in approximately 7–10% of AML cases, remains unclear.6 It is well established that FLT3 mutation status can change during the natural course of the disease in a process known as clonal evolution.6,7 With increased emphasis on genetic factors, novel treatments targeting driver mutations have been developed and have shown efficacy in clinical trials.8
Multiple studies have established the role of FLT3 inhibitors in both newly diagnosed and relapsed/refractory FLT3-mutated AML. For example, the Phase III RATIFY trial demonstrated that the addition of midostaurin to standard 7+3 induction chemotherapy significantly improved OS in FLT3-mutated AML compared to chemotherapy alone (median OS: 74.7 months versus 25.6 months; hazard ratio: 0.78; p=0.009).9 In the relapsed/refractory setting, the ADMIRAL trial showed that gilteritinib, a selective FLT3 inhibitor, significantly prolonged OS compared to salvage chemotherapy (median OS: 9.3 versus 5.6 months; hazard ratio: 0.64; p<0.001) and had a more favourable safety profile.10 These findings have led to the incorporation of FLT3 inhibitors into standard treatment protocols, and highlight the clinical benefit of targeting FLT3-driven disease biology.
Treatment selection in AML now depends on early and accurate identification of specific driver mutations.6 However, the growing number of modern diagnostic techniques and the wide range of novel diagnostic algorithms result in significant variations across laboratories in the UK, including differences in access to investigations and turnaround times for results.8
This study employed the Delphi consensus technique to gather expert opinions aimed at supporting the optimal care of FLT3-mutated AML in the UK.
METHODS
The process employed a modified Delphi methodology.11 The process is presented in Figure 1 and is described in this section.
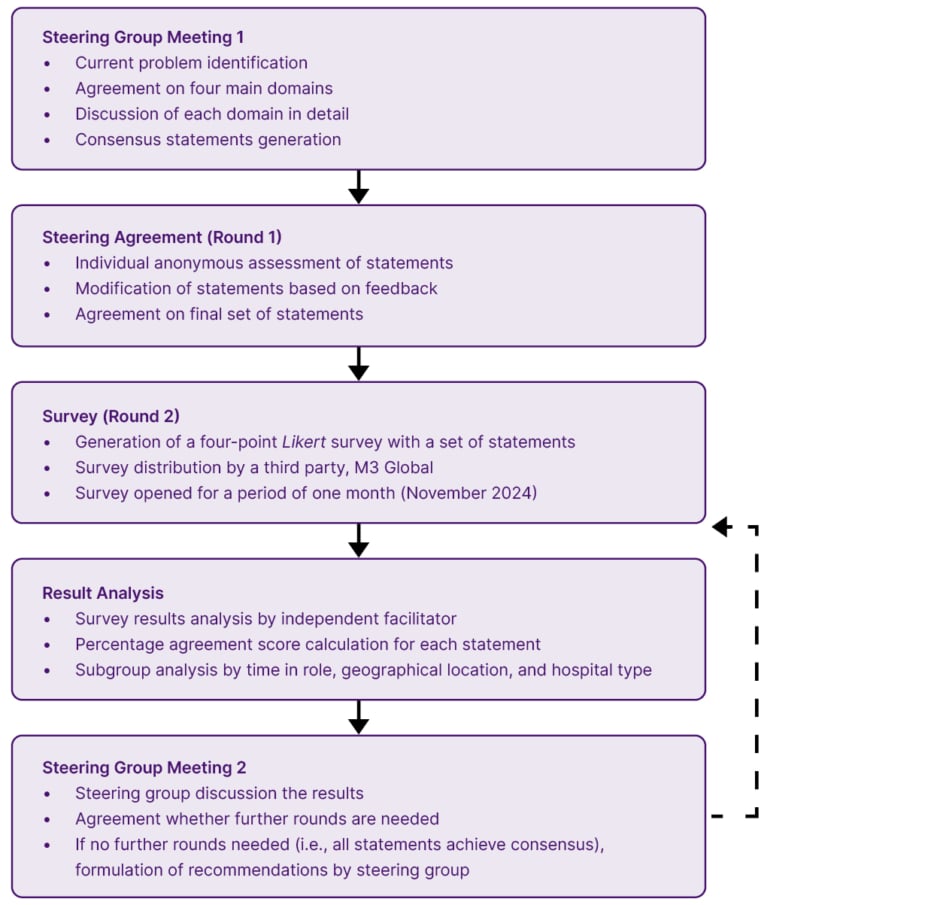
Figure 1: Modified Delphi study design.
A literature review on the optimal care of AML, encouraging specific access and defining patient care outcomes in the UK, was conducted on PubMed and Google Scholar in July 2024. Articles, research papers, conference materials, and other resources were checked for their relevance to the theme of the current study. A general web search using free text terms was also performed to locate any relevant literature sources. This additional search aimed to identify any information not indexed in the selected databases. Search terms included, but were not limited to, ‘acute myeloid leukaemia’, ‘myeloid leukaemia’, and ‘UK’, as related phrases were incorporated to maximise results.
Guided by an independent facilitator (Triducive Partners Ltd, Royal Tunbridge Wells, UK), a steering group of 10 UK consultant haematologists/haemato-oncologists, specialising in the management and treatment of AML, convened in September 2024 to discuss the problem. The steering group members were selected based on their areas of expertise and experience in the management of AML, previous publications, years of professional experience, and location. The information gathered from the literature review was used to inform the meeting discussion. As part of the discussion, the group agreed on four main domains of focus:
- Defining the optimal role and timing of testing for patients with AML, including FLT3 status testing and retesting at relapse or determination of refractory disease.
- Determining which patients are unfit/unsuitable for intensive chemotherapy.
- Defining and treating patients who are FLT3+, unfit for chemotherapy, and who are refractory or relapse.
- Defining the position of gilteritinib as a treatment option for patients with FLT3+ mutated AML.
These domains were each discussed by the steering group during the initial meeting, and 36 statements were initially agreed by the group. These statements were then collated and independently rated by the group members as either ‘accept’, ‘remove’, or ‘reword with suggested changes’. Recommendations were accepted based on a simple majority. This constituted the initial round of consensus. At the end of this process, 31 statements were finalised for testing with a wider panel of experts during the second round.
In Round 2, a 4-point Likert survey was used, allowing responses such as ‘strongly disagree’, ‘tend to disagree’, ‘tend to agree’, and ‘strongly agree’. The survey was developed and shared with a broader audience of healthcare professionals (HCP) by the independent agency M3 Global, London, UK. M3 Global Research is a market research organisation with the world’s largest database of physicians and caregivers. The database includes over four million physician members in the USA, Asia, and Europe, who signed up voluntarily to participate in the surveys. With prior written consent, the survey was distributed among HCPs with the appropriate specialty interests in the UK in accordance with the study’s objectives and inclusion criteria. The survey took an average of 20 minutes to complete. The identities of individual respondents were not known to the steering group or to the independent facilitator, ensuring the anonymity of the survey. No personal information beyond demographic data (current role, time in the current role, country, and hospital type) was captured during the survey. As this study only collected the anonymous opinions of HCPs, and no patient-specific data were captured, ethical approval was not sought.
All survey respondents resided and practiced in the UK in the area of haematology. Participants were required to be experienced in managing and treating patients with AML. A consent statement was included at the start of the survey, and each respondent indicated their agreement to participate by filling out and submitting the questionnaire. All collected responses were incorporated into the final analysis.
Stopping criteria were established a priori as a 1-month period to collect responses, a target of 100 responses, 90% of statements passing the threshold for consensus, and a threshold for consensus set at 75% (a widely accepted threshold).12 These criteria were established to gain the required number of responses while accounting for time pressures within the healthcare system. The Round 2 survey was open for 1 month from November 2024. Since the stopping criteria were met at Round 2, no more rounds of the Delphi consensus were conducted.
Completed surveys were collated and analysed by the independent facilitator to produce an arithmetic agreement score for each statement using Microsoft Excel (Microsoft, Redmond, Washington, USA) software. This was then reviewed by the members of the steering group to determine what recommendations and conclusions could be made in line with the agreement levels achieved. Analysis of Round 2 was carried out in November 2024, and the steering group reconvened on the 24th of November 2024 for analysis and discussion of the results. To present the findings of the current study, ACcurate COnsensus Reporting Document (ACCORD) guidelines were adhered to.13
Patient and Public Involvement
There was no patient or public involvement. The stated objective was to examine the opinions of HCPs towards practical considerations supporting the optimal care of AML.
Data availability statement
Data is available on reasonable request from Triducive Partners Ltd.
RESULTS
During the first round of consensus testing, 10 statements were modified, and five statements were removed, resulting in a final set of 31 statements for testing with the wider survey panel during Round 2. Completed Round 2 surveys were received from 100 haematologists with experience in the management of patients with AML. All questionnaires were included in the final analysis.
The responder group had considerable experience in managing AML: 16 had more than 20 years of experience, 45 reported 11–20 years, 31 had 6–10 years, and eight respondents reported ≤5 years of experience in their role (Supplementary Figure 1).
The majority of respondents were based in England (n=87), while nine respondents were from Scotland, and two respondents each were based in Wales and Northern Ireland (Supplementary Figure 2). The majority of respondents were based in a tertiary (specialist) centre (n=60), with 24 based in a Level 2 centre (defined as a hospital with Level 2 Critical Care facilities), and 16 based in a district general hospital (Figure 2, Supplementary Figure 3).
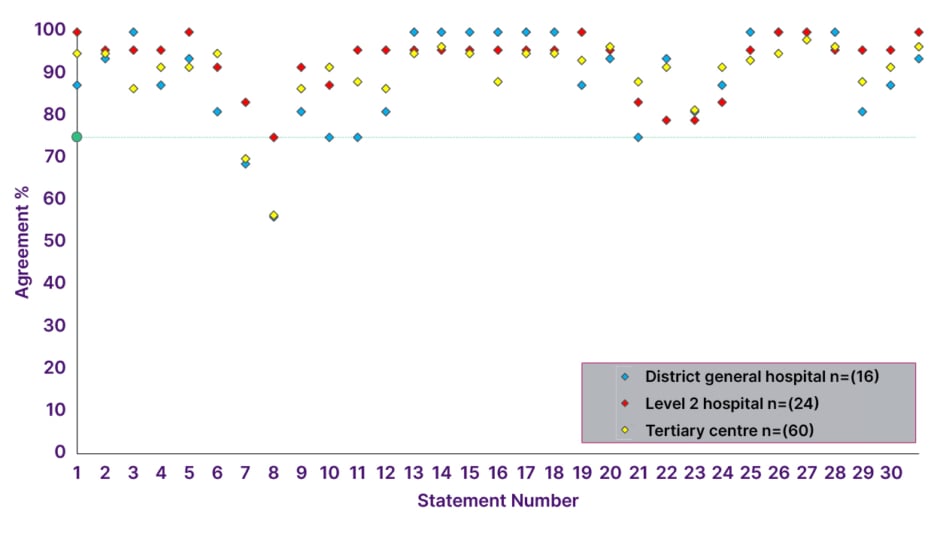
Figure 2: Consensus agreement levels by hospital type.
The threshold for consensus is depicted by the green line (75%). Level 2 is defined as a hospital with Level 2 Critical Care facilities (also known as High Dependency Units). Tertiary centres provide highly specialised treatment for conditions that are not typically managed at the local level.
In Round 2, consensus was achieved in 29 statements (94%), with 20 statements achieving over 90% agreement, and two statements failing to achieve consensus (Table 1, Figure 2).
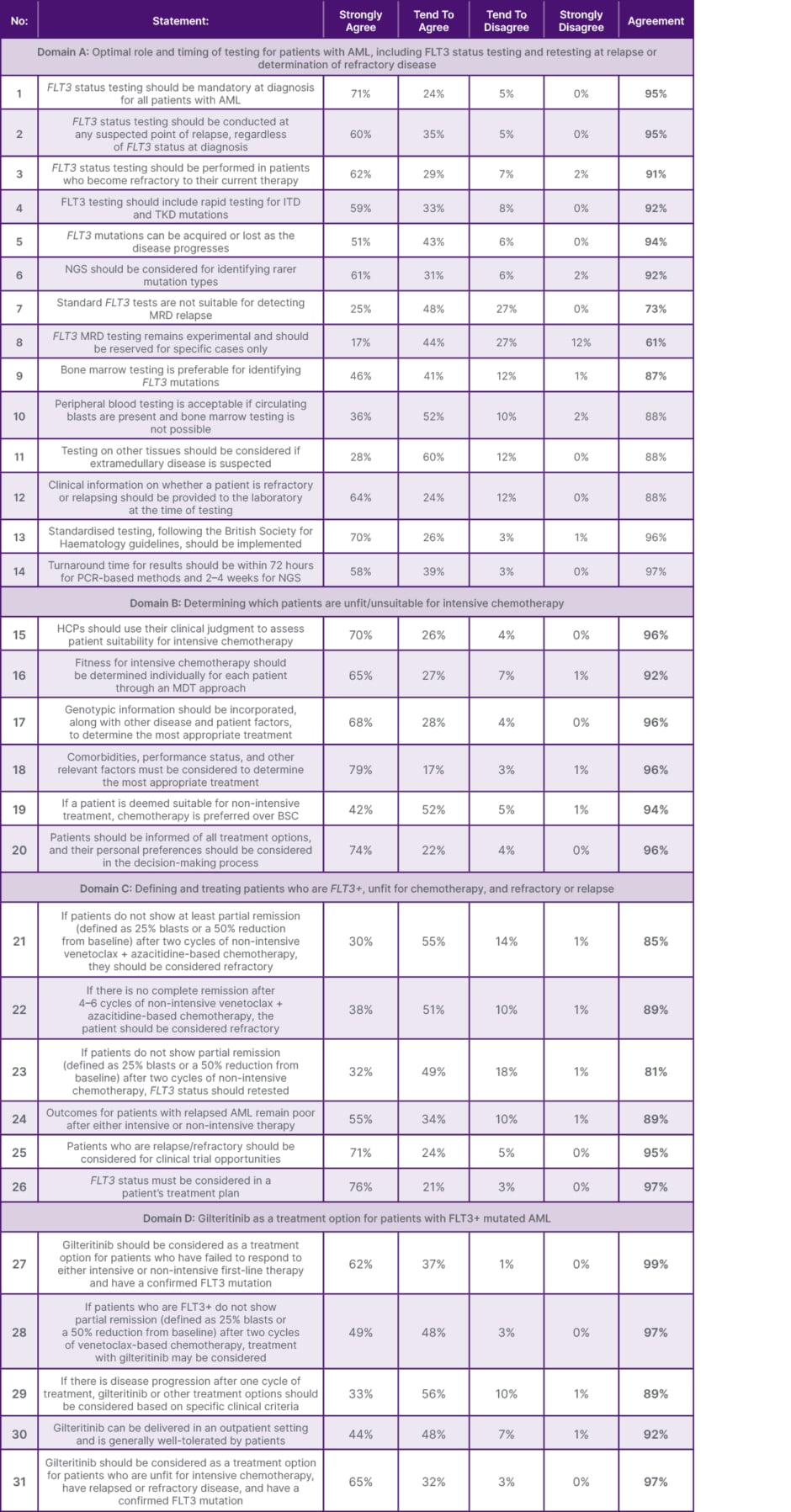
Table 1: Defined consensus statements and corresponding levels of agreement (all numbers rounded to the nearest whole number).
AML: acute myeloid leukaemia; BSC: best supportive care; HCP: healthcare professional; ITD: internal tandem duplication; MDT: multidisciplinary team; MRD: measurable residual disease; NGS: next-generation sequencing; TKD: tyrosine kinase domain.
Distribution of consensus scores on the 4-point Likert scale provided to respondents is represented in Supplementary Figure 4.
Agreement levels by hospital setting are shown in Figure 2.
Domain A: Defining the Optimal Role and Timing of Testing for Patients with AML, Including FLT3 Status Testing and Retesting at Relapse or Determination of Refractory Disease
For this domain, Round 2 of the process reached consensus that FLT3 status testing should be mandatory at the time of diagnosis (Statement 1 [S1], 95%). It is also essential to conduct FLT3 testing at any suspected point of relapse, irrespective of the FLT3 status at diagnosis, and in patients who become refractory to their current therapy (S2, 95% and S3, 91%). The testing process should include rapid screening for ITD and TKD mutations, as FLT3 mutations can be acquired or lost during disease progression (S4, 92% and S5, 95%). For the detection of rarer mutation types, next-generation sequencing (NGS) should be considered (S6, 92%).
The following two statements failed to achieve consensus: “standard FLT3 tests are unsuitable for detecting measurable residual disease (MRD) relapse” and “FLT3 MRD testing remains experimental and should only be used in specific cases” (S7, 72% and S8, 61%, respectively). MRD testing should be discussed with specialist laboratories offering MRD services, as this test is not standardised and not all laboratories can perform it. Bone marrow samples are the preferred specimen for FLT3 mutation testing (S9, 86%). However, if bone marrow sampling is not possible, peripheral blood testing, including whole blood or purified blood mononuclear cells, is acceptable, provided circulating blasts are present (S10, 89%). If extramedullary disease is suspected, other tissue types should also be considered, as sites such as skin, central nervous system, lymph nodes, spleen, and others may be involved (S11, 88%).
It is essential for laboratories to be informed about the clinical status of the patient at the time of testing, particularly if the patient is refractory or experiencing a relapse (S12, 88%). All FLT3 testing should adhere to the guidelines set by the British Society for Haematology (BSH) (S13, 96%). Additionally, the turnaround time for results should be within 72 hours for PCR-based methods and 2–4 weeks for NGS (S14, 97%).
Domain B: Determining which Patients are Unfit/Unsuitable for Intensive Chemotherapy
HCPs should assess the suitability of patients for intensive chemotherapy using clinical judgment and a multidisciplinary team (MDT) approach (S15, 97% and S16, 91%). Treatment decisions should consider genotypic information, disease characteristics, patient factors, comorbidities, geriatric assessment, and performance status (S17, 96% and S18, 96%). For patients eligible for non-intensive treatment, chemotherapy is preferred over best supportive care (S19, 95%). Patients should be informed of all treatment options, and their personal preferences must be factored into the decision-making process (S20, 96%).
Domain C: Defining and Treating Patients Who Are FLT3+, Unfit for Chemotherapy, and Refractory or Relapse
Based on the statements in this domain, a diagnostic and treatment pathway (Figure 3) was developed to improve the patient journey and outcomes.
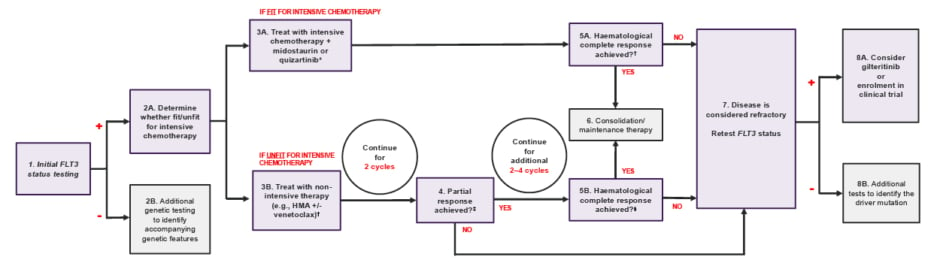
Figure 3: Acute myeloid leukaemia diagnostic and treatment pathway.
*Depending on the indication, Midostaurin is recommended for adult patients with newly diagnosed AML who are FLT3 mutation-positive.14 Quizartinib is recommended for the treatment of newly diagnosed FLT3-ITD-positive AML in adults.15
✝HMA: Hypomethylating agent such as decitabine or azacitidine.
‡Partial response defined as 25% blasts or a 50% reduction from baseline.
§Complete response defined as bone marrow blasts <5%; absence of circulating blasts; absence of extramedullary disease; absolute neutrophil count ≥1.0×109/L (1,000/µL); platelet count ≥100 × 109/L (100,000/µL).
AML: acute myeloid leukaemia; HMA: hypomethylating agent.
Patients undergoing non-intensive therapy for AML, such as venetoclax and azacitidine-based chemotherapy, should be evaluated for remission at specific intervals (S21, 85% and S22, 88%). The European LeukemiaNet (ELN) recommendations for diagnosis and management of AML in adults proposed definitions of haematological complete response (CR) and partial response (PR) that should be followed to determine patients with relapsed/refractory disease:5
- CR: Bone marrow blasts <5%; absence of circulating blasts; absence of extramedullary disease; absolute neutrophil count: ≥1.0×109/L (1,000/µL); platelet count: ≥100×109/L (100,000/µL).5
- PR: All haematologic criteria of CR; decrease of bone marrow blast percentage to between 5% and 25%; and decrease of pretreatment bone marrow blast percentage by at least 50%.5
For patients not showing a PR after two cycles of non-intensive therapy, FLT3 status should be retested (S23, 83%).7 Testing of FLT3 status must be integrated into treatment planning (S26, 97%), as some therapeutic agents, such as second-line treatment with an FLT3 inhibitor, demonstrated beneficial efficacy and safety results.16 Outcomes for patients with relapsed AML are poor after both intensive and non- intensive therapy (S24, 89%);16 therefore, clinical trial opportunities should be considered for patients who are relapsed/refractory (S25, 95%).16
Domain D: Gilteritinib as a Treatment Option for Patients with FLT3+ Mutated AML
Gilteritinib is a second-line treatment option for patients with confirmed FLT3+ AML who have failed to respond to either intensive or non-intensive first-line therapy (S27, 99%).17 It may also be considered:
- For patients who are FLT3+ and who do not achieve a PR after two cycles of venetoclax-based chemotherapy (S28, 97%).
- If disease progression is confirmed during or immediately after the first cycle of treatment, based on clinical criteria (S29, 89%).
- For any patient unfit for intensive therapy with relapsed or refractory disease and confirmed FLT3 mutation (S31, 97%), as recommended by the National Institute for Health and Care Excellence (NICE) guidelines 2020.17
Gilteritinib can be administered in an outpatient setting, and offers flexibility in patient care (S31, 97%).10,17 In a Phase III trial, the percentage of patients treated with gilteritinib who had complete remission with full or partial haematologic recovery was 34.0% (n=84/247), compared with 15.3% (n=19/124) of patients on salvage chemotherapy. In the gilteritinib group, common adverse events of Grade 3 or higher were febrile neutropenia (45.9%), anaemia (40.7%), and thrombocytopenia (22.8%).10
Recommendations
The steering group propose the following recommendations intended to support the optimal care of AML in the UK:
- FLT3 status should be tested at the time of diagnosis and retested if relapse or refractory disease is determined, as the mutational status can change, with FLT3 mutations being either gained or lost.
- The testing process should follow the BSH recommendations and include rapid screening for ITD and TKD mutations, with a turnaround time of 72 hours for PCR-based methods, and 2–4 weeks for NGS.
- Treatment decisions should be made by an MDT and should incorporate clinical judgment, considering genotypic information, disease characteristics, patient-specific factors, comorbidities, geriatric assessment, and performance status.
- FLT3 status should be retested in patients who do not show at least a PR after two cycles of non-intensive therapy or who do not achieve a CR after 4–6 cycles.
- For all eligible patients unfit for intensive therapy who have relapsed or have refractory disease and a confirmed FLT3 mutation, gilteritinib should be strongly considered as a second-line treatment option.
DISCUSSION
AML in the UK predominantly affects an older population, with a median age at diagnosis of approximately 68–70 years, similar to other Western countries but older than in many Asian and some Eastern European cohorts.2,18 This older population is more likely to have significant comorbidity, as well as poorer tolerance of intensive chemotherapy.18 In addition, the UK AML population is ethnically and socioeconomically diverse, particularly in urban centres. Certain ethnic groups may have differing AML subtype distributions, responses to treatment, or pharmacogenomic profiles, although detailed UK-specific data on this remains limited.19 This heterogeneity therefore necessitates national strategies that may not directly apply in other countries, particularly those with insurance-based or decentralised healthcare systems.
In the USA, the National Comprehensive Cancer Network (NCCN) offers broader access to FDA-approved agents such as venetoclax, gilteritinib, and enasidenib, sometimes ahead of their UK approval via NICE.20 As a consequence, patients in the USA may gain access to newer combinations earlier in their treatment journey compared to those in the UK, where practice often involves initial participation in clinical trials or managed access schemes for these agents. This suggests that HCPs in the UK should make full and appropriate use of newer agents and combinations once approved for use, drawing on early experience in other countries. FLT3 status should be tested at the time of diagnosis and retested if relapsed or refractory disease is determined, as the mutational status can change.
As FLT3 status determines the choice of first-line treatment, testing is recommended for all patients with AML at diagnosis, and retesting is advised for patients with relapsed or refractory disease.7 While MRD testing for FLT3 remains experimental, there is potential for future use in predicting disease progression and patient outcomes, and as an endpoint in clinical trials.7,21
Although not specifically included in the Delphi consensus statements (and therefore not tested with the wider panel), HCPs should consider that, within FLT3+ disease, several factors impact prognosis. These include: FLT3-ITD allelic ratio with a high allelic ratio status being associated with poor prognosis, higher relapse rates, and shorter OS; the presence of multiple distinct FLT3-ITD clones; ITD insertion site and length; and the presence of co-occurring mutations (such as NPM1 or DNMT3A).5, 22-25 Where genomic data is available, it should be factored into treatment decisions.
The testing process should follow the BSH recommendations and include rapid screening for ITD and TKD mutations, with a turnaround time of 72 hours for PCR-based methods, and 2–4 weeks for NGS.
The introduction of modern diagnostic methods and techniques led to significant heterogeneity and regional variations in laboratory practices across the UK, including disparities in access to investigations, and turnaround times.8 The BSH Good Practice paper published in 2023 recommends standardising laboratory practices for AML assessment to accelerate access to appropriate treatment options and improve patient outcomes.8
FLT3 mutation testing should include rapid screening for ITD and TKD variants for all patients with AML, as both may be responsive to FLT3 inhibitors, making prompt identification crucial.7,8 Additionally, NGS panel testing is recommended for the detection of pathogenic variants, including CEBPA, TP53, FLT3, IDH1, IDH2, ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, DNMT3a, WT1, and ZRSR2. Despite the absence of a standardised NGS panel, testing should meet the minimum standards of practice and requirements defined by local or national testing algorithms.8
Timely testing is essential to expedite diagnosis and treatment access for patients with AML, with specific turnaround times detailed in the BSH guidelines.8
Treatment decisions should be made by an MDT and should incorporate clinical judgment, considering genotypic information, disease characteristics, patient-specific factors, comorbidities, geriatric assessment, and performance status.
In the process of treatment decision-making, all patients should be assessed for their suitability for intensive and non-intensive therapy at the point of diagnosis.26,27 Comprehensive fitness assessment should include genotypic information, disease characteristics, patient-specific factors, comorbidities, geriatric assessment, and performance status.26,27 These factors should be considered together, and the treatment decision should be agreed upon by an MDT.26 The MDT can include advanced practice providers, nurses, pathologists, pharmacists, navigators, and social workers. As part of this process, patient preferences and treatment goals should be considered.28
FLT3 status should be retested in patients who do not show a PR after two cycles of non-intensive therapy or who do not achieve a CR after 4–6 cycles.
Treatment response should be assessed during or immediately after treatment cycles, in alignment with recommendations by the ELN 2022.5 If relapse or refractory disease is determined, retesting should be performed to detect acquired mutations.7,8 For all eligible patients unfit for intensive therapy who have relapsed or refractory disease and a confirmed FLT3 mutation, gilteritinib should be considered as a second-line treatment option.
Gilteritinib is a highly selective oral FLT3 inhibitor with inhibitory activity against both FLT3 mutation subtypes, ITD and TKD, and the tyrosine kinase AXL, which is involved in resistance to FLT3 inhibitors, with weak activity against c-Kit.10
In ADMIRAL, gilteritinib demonstrated significantly longer survival and a higher number of remissions achieved by patients with relapsed or refractory FLT3+ AML in comparison with salvage chemotherapy.10 Gilteritinib is recommended by NICE for relapsed or refractory FLT3+ AML in adults.17
The proposed patient pathway is intended to support the standardisation of current diagnosis and treatment practice for AML in the UK, and to assist treatment decisions.
STRENGTHS AND LIMITATIONS
The strength of this study is the high level of agreement demonstrated by a representative large cohort of haematologists experienced in treating AML from the UK in almost all statements. The respondent group included a well-balanced representation from a wide range of hospitals. However, high agreement levels may indicate a lack of challenge or a bias towards agreeability. Additional bias should be considered due to the high level of representation of HCPs practising in England (n=87/100), hampering meaningful comparison of responses by devolved nations.
CONCLUSION
This modified Delphi consensus study allowed experts in haematology to develop a set of recommendations regarding the optimal care of AML in the UK. FLT3 status testing is essential at diagnosis and upon relapse or detection of refractory disease. Testing should adhere to BSH guidelines, including rapid screening for ITD and TKD mutations with specified turnaround times. Treatment decisions should be made by an appropriate MDT, incorporating clinical judgment alongside genotypic information, disease characteristics, comorbidities, geriatric assessment, and performance status.
| Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Astellas Pharma Ltd. on 0800 783 5018. |
Please scan or click for full prescribing information.
MAT-GB-XOS-2025-00058
July 2025







