Abstract
Topiramate is a well-known medication used for migraine and epilepsy. Bleeding is a rare and less documented side-effect of this treatment. The authors present a rare case of bleeding in a patient who was started on topiramate, which was likely due to a vitamin K-dependent factor deficiency. Clinicians should take caution if any bleeding event is noted in patients on topiramate, and stop administering this drug.
Key points
1. Coagulopathy is a rare side effect in topiramate.2. Though the mechanism is unclear, vitamin K dependant factor deficiency is seen in coagulation workup.
3. Treatment involves discontinuation of topiramate and fresh frozen plasma.
INTRODUCTION
Bleeding disorder is a rare and serious side effect of anticonvulsant drugs (ACD). Topiramate is a common ACD which has demonstrated good efficacy and safety in several randomised clinical trials and can be associated with coagulopathy. There are multiple mechanisms of action through which topiramate works. It can act through sodium and calcium channels, affect GABA-A receptor activity, and cause CYP3A4 enzyme induction. It is effectively used in epilepsy and migraine prophylaxis. The mechanism of coagulopathy, however, is unclear.
Vitamin K is a fat-soluble vitamin that acts as a cofactor for γ-glutamyl carboxylase, which is essential for formation of several clotting factors and anticoagulant proteins. Vitamin K deficiency can affect the synthesis of these clotting factors and can cause bleeding diathesis in patients. Very few reported cases of topiramate-associated coagulopathy have been reported in literature review. The authors present a case of vitamin K-dependent factor deficiency as a likely cause of bleeding in a patient treated with topiramate, and subsequent resolution of vitamin K deficiency after stopping topiramate and receiving fresh frozen plasma. This case identifies the importance of considering ACD-induced vitamin K-dependent factor deficiency in patients with bleeding.
PATIENT INFORMATION
The patient is a 68-year-old female with past medical history of diabetes, transient ischaemic attack, and coronary artery disease, who presented to the haematology clinic for evaluation of recently diagnosed coagulopathy. A few months ago, she was found to have thickening of the uterus and uterine polyps, for which she underwent hysteroscopy with dilatation and curettage of uterus. On the 6th post-operative day, she developed significant vaginal bleeding, and 1 week later, she had large ecchymotic areas and blood-filled blisters in her mouth. She also had a few episodes of epistaxis that were self-limited, and had to come to the emergency for further evaluation. Six years prior, she had an episode of deep vein thrombosis and underwent thrombectomy. She had one episode of bleeding after thrombectomy. After the procedure, she was treated with warfarin for 4 months and had international normalised ratios (INR) of 8–9s while being on warfarin therapy. An inferior vena cava filter was placed and warfarin was discontinued.
The patient had started topiramate for migraine a few weeks before surgery. Although topiramate was stopped a week prior to surgery, it was resumed postoperatively, and the patient was on topiramate at the time of bleeding. Other medications she was taking during the hospital visit were cholecalciferol, colesevelam, eszopiclone, febuxostat, furosemide, lansoprazole, magnesium, metoprolol succinate, multivitamin, omega-3 acid, potassium chloride, riboflavin, and venlafaxine, which were her chronic medications.
CLINICAL FINDINGS AND DIAGNOSTIC ASSESSMENT
Her labs (Table 1) showed severely elevated prothrombin time (PT)/INR and partial thromboplastin time (pTT; PT >124, INR >9.9, pTT >350) while her d-dimer (320), fibrinogen (351), and platelet counts (212,000) were all normal. Labs showed that factor VIII (162) level was normal, but factors II (58),VII (18), and X(41) were low. Warfarin level and von Willebrand factor multimers (146) were normal. Antiphospholipid, cardiolipin, and β2 glycoprotein antibodies were also negative. She was given four units of fresh frozen plasma at the time of the emergency, with improvement of her PT (16.2), INR (1.6), and pTT (75.9). Mixing studies showed normalisation of PT and pTT, consistent with coagulation factor deficiency, and ruled out the presence of any inhibitor.
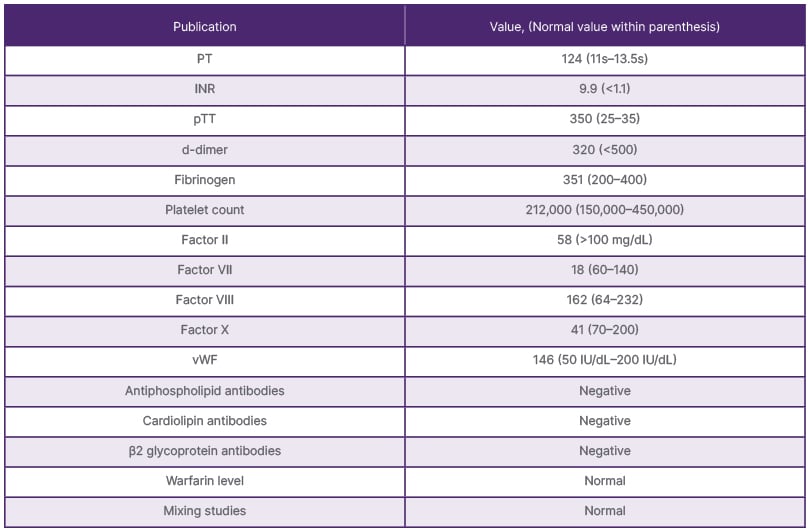
Table 1: Patient’s lab results before fresh frozen plasma was given.
INR: international normalised ratio; PT: prothrombin time; pTT: partial thromboplastin time; vWF: von Willebrand factor.
THERAPEUTIC INTERVENTION
Topiramate was discontinued during the hospitalisation for concerns of coagulopathy. The patient received fresh frozen plasma, causing reversal of her coagulopathy.
FOLLOW-UP AND OUTCOMES
After stopping topiramate, she did have some minor vaginal bleeding, which stopped at follow-up visits. She also denied any new ecchymosis or epistaxis. After a few weeks, she was restarted on topiramate and she again had abnormal blood parameters. Her PT was 30.8, INR was 3.1, and activated pTT (apTT) was 34.7. The levels normalised when the topiramate was stopped permanently. Topiramate was replaced with valproic acid for her migraine. Her PT/INR and pTT levels were monitored regularly and have remained normal since stopping topiramate.
DISCUSSION
Vitamin K is an essential vitamin for the function of coagulation factors II, VII, IX, X, and the anticoagulants protein C and S.1 The causes of vitamin K-dependent factor deficiency are malabsorption, dietary deficiency, liver disease, and drug interactions.2 The symptoms related to deficiency include bleeding, epistaxis, ecchymosis, vaginal bleeding, and rectal bleeding. The laboratory abnormalities that can be seen due to vitamin K-related factor deficiency are prolonged PT and aPTT, owing to deficiency of factors II, VII, IX, and X. Coagulopathy with the increase in PT and aPTT can also be caused by liver diseases, anti-coagulant use/toxins (inhibitors), disseminated intravascular coagulation, antiphospholipid antibodies, and a variety of inherited coagulation deficiencies. The changes in PT and aPTT due to factor deficiency versus inhibitors can be seen in Table 2.
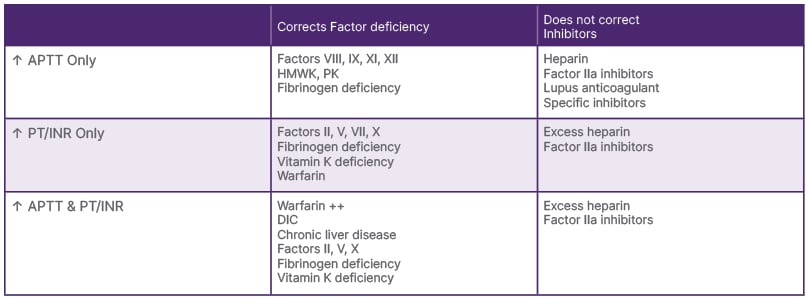
Table 2: Changes in prothrombin time and partial thromboplastin time due to factor deficiency versus inhibitors.
APTT: activated partial thromboplastin time; DIC: disseminated intravascular coagulation; HMWK: high molecular weight kininogen; INR: international normalised ratio; PK: prekallikrein; PT: prothrombin time.
Topiramate is an ACD that is used for prophylaxis of migraine attacks and is approved for partial and generalised tonic-clonic seizures.3,4 Common side-effects of topiramate include fatigue, dizziness, cognitive dysfunction, metabolic acidosis, etc. Anticonvulsants have been shown to cause bleeding disorders and inhibit vitamin K activity. It has been suggested that ACD consumption in pregnancy can cause bleeding disorders in the mother and newborn.5 Some case reports, case series, and small cohorts from birth registries have raised concerns of an elevated risk of obstetric bleeding complications in females on ACD, but the results are inconsistent.6-9 The possible cause mentioned is that ACDs easily cross the placenta and induce microsomal enzymes in the fetal liver, which will lead to deficiency of vitamin K and reduced concentration of vitamin K-dependent factors.10 It should be noted that topiramate is a weak inducer of CYP3A4 microsomal enzyme, which is a key player in the body’s metabolism of drugs.11 It can decrease the effects of non-vitamin K antagonist oral anticoagulants (NOAC) by inducing CYP3A4 activity. Topiramate can also cause hepatotoxicity, which may contribute to decreased synthesis of clotting factors.12
Two case reports with epistaxis in a patient on topiramate were found during the literature search. Polimeni et al.13 reported a case of epistaxis in a 36-year-old female taking topiramate for migraine. The patient was advised to stop taking topiramate and the bleeding stopped 12 hours later.13 Another article by Page et al.14 mentioned epistaxis in a 61-year-old female patient who was prescribed topiramate 25 mg daily, and who developed epistaxis 7 days after treatment, which improved after stopping the topiramate.14 Both the articles suggested anti-platelet effects as a possible mechanism of topiramate, particularly in combination with antiplatelet medication. Wang et al.15 showed that when topiramate was combined with a NOAC, it was not associated with higher risks of major bleeding (crude major bleeding incidence rate of 55.16% per 1,000 person years; risk ratio of 1.09 compared to a NOAC).15 Perrone et al.16 presented two cases of vitamin K–dependent clotting factor deficiency with pathogenic variants in the GGCX gene, where one patient exhibited severe early-onset bleeding manifestations, including intracranial haemorrhage, necessitating frequent plasma infusions and high-dose vitamin K, while the other displayed a milder phenotype characterised by mucocutaneous bleeding, and responded favourably to intermittent vitamin K therapy.16
In the authors’ case, the patient was on topiramate and had bleeding abnormality consistent with vitamin K-dependent factor deficiency evident by low factor II, VII, and X levels, which was corrected by giving fresh frozen plasma and stopping topiramate.
DIFFERENTIAL DIAGNOSIS
Her coagulopathy could have been from vitamin K-dependent factor deficiency due to warfarin; however, she denied any recent warfarin use. Warfarin poisoning was also considered as her husband had been on warfarin, but her warfarin level was normal. She also denied any accidental rat poison intake. She mentioned taking a standard diet with lots of salad, which made a nutritional cause highly unlikely.
STRENGTHS AND LIMITATIONS
Topiramate induced bleeding is a rare complication and only a few reports have been found in the literature, as mentioned previously. The authors’ case report can alert clinicians on this rare adverse event of topiramate, and help understand the timeline, laboratory findings, and management strategies. There were no possible confounders that would have caused the bleeding; however, the mechanism of the bleeding is not completely clear. Further studies to assess the bleeding risk are needed.
CONCLUSION
In conclusion, bleeding due to vitamin K-dependent factor deficiency is a rare side effect of topiramate. The possible mechanism is unclear. Careful use of topiramate by clinicians is recommended in patients with bleeding disorders, and prompt recognition and discontinuation of the drug can lead to resolution of symptoms and normalisation of coagulation parameters.







