Abstract
Synchronous twin malignancies, defined as the simultaneous occurrence of two distinct primary cancers in the same patient, represent a rare and challenging clinical scenario. Synchronous diagnosis of haematological and solid malignancy poses a challenge in treatment planning. This case study details the presentation, diagnosis, treatment, and eventual outcome of a 32-year-old female with acute promyelocytic leukaemia and metastatic non-small cell lung carcinoma. The patient’s complex clinical course, including the development of pancytopenia with bleeding manifestations, therapy-related issues, and progressive disease, underscores the need for a high index of suspicion in managing such rare oncological presentations.
Key points
1. Dual presentations of haematologic and solid tumours remain extremely uncommon. The literature notes dismal outcomes for synchronous acute myeloid leukaemia‑solid tumour cases, with a median overall survival of just 3.6–20.0 months.2. Synchronous acute promyelocytic leukaemia and non-small cell lung carcinoma is extremely rare (≈0.2–1.5%) and is consistently associated with poor prognosis.
3. New or persistent symptoms in a patient with cancer who was treated warrant thorough imaging and molecular testing to detect possible second primaries.
INTRODUCTION
Multiple primary cancers are not an unusual phenomenon in current oncology practice. These are commonly metachronous (more than 6 months between the diagnoses of two malignancies) or less commonly synchronous (within 6 months of the first primary diagnosis).1-3 There are many cancer predisposition syndromes in which the sequential occurrence of different types of primary malignancies has been reported.1-5
There are limited data on synchronous twin malignancies. The simultaneous occurrence of haematologic and solid tumours presents unique diagnostic and therapeutic challenges, often requiring an integrated approach for management.5-12 This case report discusses a rare presentation of synchronous acute promyelocytic leukaemia (APML) and non-small cell lung carcinoma (NSCLC) in a young female, focusing on the clinical complexities and treatment strategies employed.
CASE PRESENTATION
A 32-year-old female, with a medical history notable only for treated tuberculosis with pleural effusion 3 years prior, presented with breathlessness, ecchymotic patches on her skin, and increased per vaginum bleeding lasting 3–4 days. On examination, there was no organomegaly or lymphadenopathy. A complete blood count revealed pancytopenia (complete blood count: 4.8/4,890/8,000, P35L30M35), prompting her admission to the ICU due to signs of heart failure.
Peripheral smear analysis showed the presence of Faggot cells and abnormal promyelocytes, comprising 30–32% of the cell population (Figure 1). The smear tested positive for myeloperoxidase (MPO). While liver and kidney function tests remained normal, her coagulation profile was abnormal, with an activated partial thromboplastin time of 46 seconds (control 33 seconds) and low fibrinogen levels (152 mg/dL).
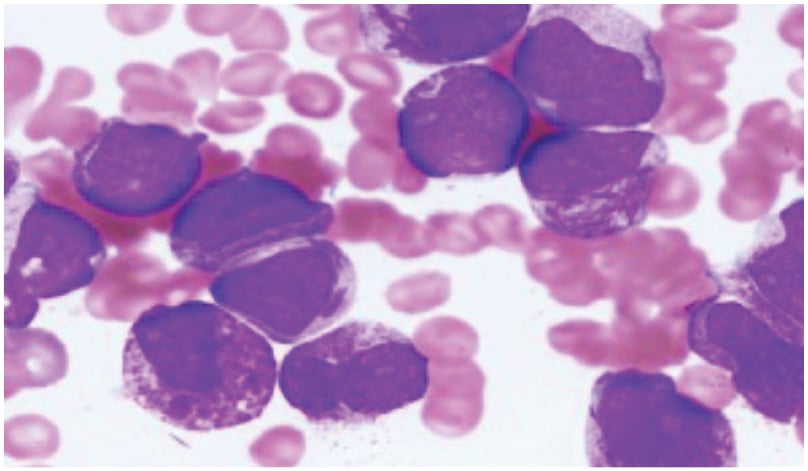
Figure 1: Bone marrow biopsy showing hypercellular marrow with 80–90% abnormal promyelocytes, with large nuclei, Auer rods, and Faggot cells.
Her BMA/Bx was performed immediately, which revealed hypercellular marrow with 80–90% abnormal promyelocytes, large nuclei, open chromatin, markedly coarse granular cytoplasm (hypergranular) with prominent to inconspicuous nucleoli, a few Auer rods, and a few lobulated nuclei.
Flow: positive for CD38, CD33, CD117, HLA-DR, CD64, and MPO.
BMA: bone marrow aspiration; Bx: biopsy; CD33: cluster of differentiation 33; CD38: cluster of differentiaton 38; CD64: cluster of differentiation 64; CD117: cluster of differentiation 117; HLA-DR: human leucocyte antigen DR; MPO: myeloperoxidase.
Bone marrow aspiration and biopsy indicated hypercellular marrow with 80–90% abnormal promyelocytes characterised by large nuclei, open chromatin, markedly coarse granular cytoplasm (hyper granular) with prominent to inconspicuous nucleoli, and a few Auer rods (Figure 1). Fluorescence in situ hybridisation confirmed the presence of 98% of cells positive for the t(15,17) PML-RARA fusion gene, leading to a diagnosis of APML. Initial high-resolution CT and PET-CT scans revealed multiple mildly fluorodeoxyglucose (FDG)-avid patchy areas of ground-glass opacities in both lungs, an FDG-avid nodular lesion in the left upper lobe (2.2 cm), and an FDG-avid mass in the left pre-vascular area (3.2 cm; Figure 2).
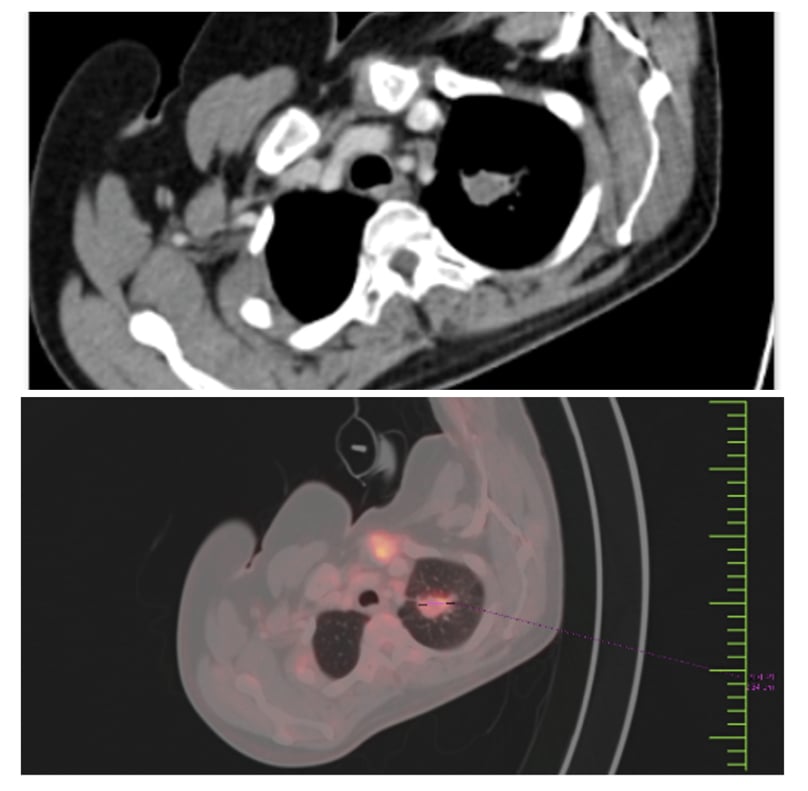
Figure 2: PET-CT and CT chest at baseline.
Multiple mildly FDG-avid patchy areas of GGOs in both lungs.
FDG-avid nodular enhancing lesion in left upper lobe; 1.2 cm; SUV: 11.
FDG-avid enhancing nodal mass in left prevascular area: 2.2 cm; SUV max: 9
No skeletal lesions.
FDG: fluorodeoxyglucose; GGO: ground-glass opacity; SUV: standardised uptake value.
TREATMENT OF ACUTE PROMYELOCYTIC LEUKAEMIA
She was started on arsenic trioxide (ATO) and all-trans retinoic acid (ATRA) therapy, accompanied by supportive care. She received Lo-coco protocol induction ATO (0.15 mg/kg dose) and ATRA (45 mg/m2) for 45 days, along with posaconazole and valacyclovir prophylaxis for fungal and herpes infection, respectively, from Day 1 of therapy.
The patient tolerated that well, with differentiation syndrome managed with hydroxyurea and dexamethasone. Her bone marrow aspiration and biopsy after morphologically normal complete blood count reports were done after 45 days of ATO + ATRA, and she was in complete remission. She did not have any pseudotumor cerebri with ATRA during the therapy, and her real-time quantitative polymerase chain reaction (RQPCR) for PML RARA was 0.024% at the end of induction.
DIAGNOSIS OF SYNCHRONOUS MALIGNANCIES
She was started on the Lo-coco consolidation protocol with ATO and ATRA for 28 weeks. While the patient was undergoing consolidation therapy, she developed persistent headaches. Both an MRI of the brain and her cerebrospinal fluid analysis for malignant cells and cytology were normal. ATRA was stopped from Week 8 of consolidation therapy for ATRA-induced pseudotumor, and she was started on acetazolamide tablets. She continued to have a gradually worsening headache despite anti-cerebral oedema measures. Her repeat RQPCR for PML RARA was 0.0%, confirming the disease was in remission.
Given the persistent headaches, MRI of the brain and spine revealed multiple lytic lesions in the cervical spine, suggestive of metastatic disease. A repeat PET-CT scan identified new FDG-avid lesions in the liver and bones, raising suspicion for a second primary malignancy (Figure 3).
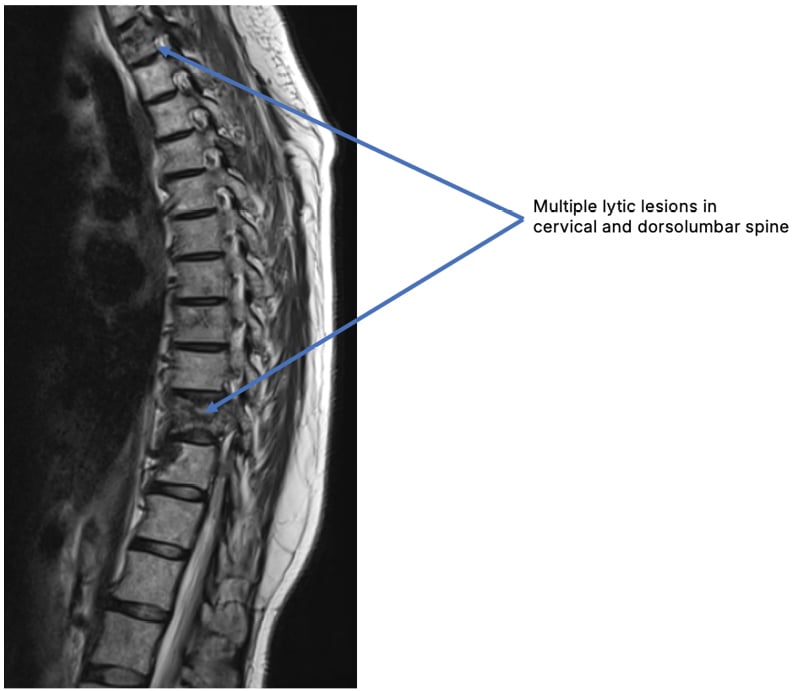
Figure 3: Multiple lytic lesions in the cervical spine with no nerve root or cord compression.
CT-guided biopsy of the liver lesion revealed carcinosarcoma with adeno-carcinomatous and sarcomatoid components (Figure 4). Immunohistochemistry confirmed the presence of adenocarcinoma cells positive for CK7, CK19, TTF1, and napsin, with sarcomatous cells positive for vimentin and P53. Genetic profiling by next-generation sequencing detected an EML4-ALK mutation, consistent with NSCLC.
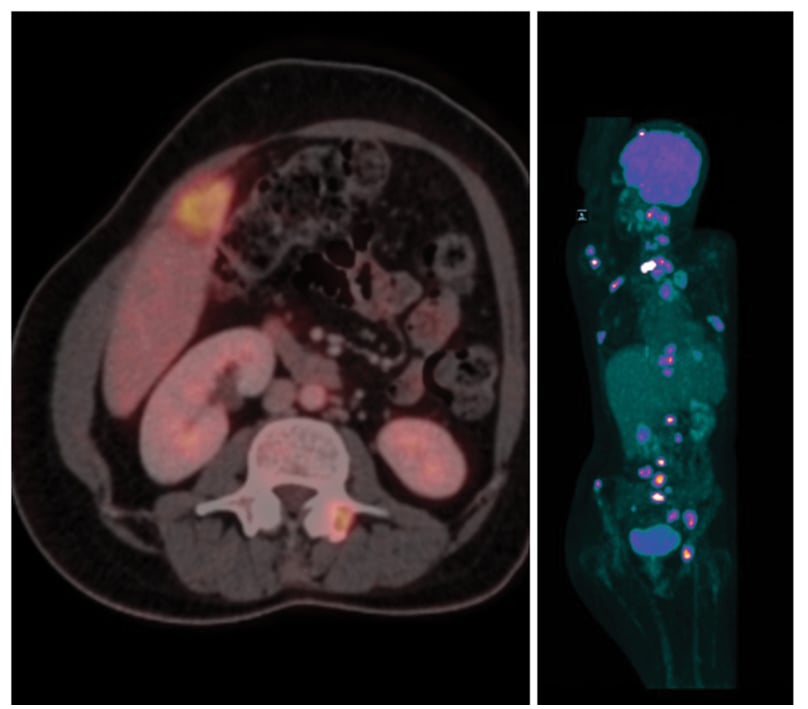
Figure 4: PET-CT showing extensive disease metastases.
FDG-avid left supra-clavicular node: 2.5 cm; Well-defined FDG-avid left upper lobe lung mass: 2.6 cm; Few small FDG-avid nodules in both lobes of lung; largest: 1.2 cm; FDG-avid prevascular LN mass: 4.2 cm; FDG-avid lesion in right lobe of liver: 3.2 cm.
Lytic lesions involving skull vault, base of skull, multiple cervical vertebrae, bilateral humerus, left clavicle. Multiple dorsolumbar vertebrae, sacrum, acetabulum suggestive of metastases from possible lung cancer.
FDG: fluorodeoxyglucose; LN: lymph node.
TREATMENT AND DISEASE PROGRESSION
The patient underwent a complex treatment regimen tailored to both malignancies. For APML, she received ATO and ATRA-based induction and consolidation therapies. Due to the development of ATRA-induced pseudotumour cerebri, ATRA was discontinued after 8 weeks of consolidation. Her APML therapy was stopped after diagnosis of NSCLC, as APML was in remission (RQPCR for PML RARA was 0.0%) while she had metastatic NSCLC.
For NSCLC, she received chemotherapy with paclitaxel and carboplatin while the immunohistochemistry report was awaited for debulking therapy, followed by targeted therapy with alectinib for the ALK-positive mutation. She underwent radiofrequency ablation for the D1 lesion followed by vertebroplasty, and subsequently received external beam radiation therapy to the clivus metastasis (20 Gy/5#), which achieved pain relief.
Repeat PET-CT was suggestive of oligoprogressive disease with reduction of nodal and lung lesion, and a few new bone lesions. She then experienced worsening of back pain, and a repeat MRI was suggestive of multiple metastatic lesions with surrounding marrow oedema involving the vertebrae, with associated extraosseous soft tissue and pathological compression fractures in D1 and D10 vertebrae. She then received radiotherapy to D1 and D10, followed by four cycles of gemcitabine + docetaxel. Repeat PET-CT was suggestive of progressive disease with new sites of nodal disease and an increase in hepatic lesion in segment V of the right lobe of the liver to 16 x 15.7 cm.
She was then started on pazopanib 400 mg tablets once daily and palliative radiotherapy 20 Gy/5# to the liver lesions. She had partial response of the liver lesions but increase in paratracheal and paraesophageal nodes, with new bone lesions and peritoneal metastases. She eventually was kept only on pain management and died due to the metastases.
OUTCOME
Despite aggressive treatment, the patient’s condition deteriorated. Her final PET-CT scan showed progression of the liver and bone metastases, with new peritoneal metastases. She was transitioned to palliative care and eventually died from the disease.
DISCUSSION
Simultaneous occurrence of two primary malignancies in a single patient is a rare occurrence. It is usually associated with addictions like smoking, alcohol, or tobacco, and/or in patients with genetic risk of cancer or family history of cancers (germline mutation in the colon or BRCA in breast and ovarian cancers).
In 1879, Billroth first described multiple primary malignancies. The neoplasm may be limited to one organ or multiple different organs, and they are classified into two categories: synchronous, in which the cancers occur at the same time or within 2 months; and metachronous, in which the cancers follow in sequence, that is, more than 6 months apart.10
Metachronous primary malignancies are becoming increasingly common because the population is living longer than in the past decades, there is a high number of cancer survivors, and there is greater awareness and improved diagnostic modalities. Following chemotherapy and/or radiotherapy for solid organ cancers, occurrence of secondary malignancies after 2–10 years, such as myelodysplastic syndrome, acute myeloid leukaemia (AML) or acute/chronic lymphocytic leukaemia, is common and has been reported in literature (i.e., metachronous presentation). However, synchronous presentation of AML and solid tumours is rare.
Risk factors for AML are exposure to chemicals like benzene or radiation, and exposure to chemotherapy agents like anthracyclines or topoisomerase inhibitors.13-14 Topoisomerase II inhibitors or alkylating agents, and abnormalities of chromosomes such as 11q23, are known to cause myelodysplastic syndrome/AML in 1–10 years post-chemotherapy depending on the type of chemotherapy that is used.
Risk factors for lung cancer include smoking or tobacco, and exposure to industrial chemical fumes or asbestosis.13-14
In the literature review, the authors found that only a handful of articles have been published so far of synchronous AML with solid tumours. The outcome of all has been dismal when AML is associated with solid cancers synchronously, and none have survived so far.10-12 Vararajan11 published 12 cases of dual malignancies, of which seven patients presented with metachronous AML and lung cancer, and five patients had synchronous AML and lung cancer.11 Sheridan et al.12 published a case report of synchronous presentation of AML and breast cancer.
In a meta-analysis of 32 patients with synchronous haematological malignancies and solid tumours, there were only four cases of AML, out of which one case was of APML. Their overall survival varied between 3.6–20.0 months, with the eventual outcome being mortality. AML with synchronous solid tumours had poor outcomes, despite whatever strategies of treatment were employed.15
Treatment strategies in case of synchronous double malignancy depend on whether you are treating the malignancy that is more advanced first, or both are treated simultaneously. In the authors’ case, the NSCLC was metastatic, so the patient was treated for NSCLC rather than APML.15-18
Synchronous twin malignancies, particularly when a haematologic cancer like APML coexists with a solid tumour such as NSCLC, are exceedingly rare. This simultaneous presence of malignancies complicates both diagnosis and treatment strategies. The case underscores the necessity for comprehensive diagnostic evaluations, including advanced imaging techniques and molecular profiling, to inform treatment decisions.
Managing synchronous malignancies demands a multidisciplinary approach that balances the aggressive treatment of each cancer while addressing overlapping toxicities and complications.
Additionally, this case highlights the challenges associated with treatment-induced side effects, such as ATRA-induced pseudotumor cerebri, emphasising the importance of vigilant monitoring and timely intervention.
CONCLUSION
Synchronous twin malignancies with AML and solid cancers have a dismal outcome, due to underlying disease and delays in the diagnosis of the second cancer, which eventually metastasises. Furthermore, it is challenging to treat both malignancies at the same time. Adverse events of one therapy also complicate the other therapy options and overall outcomes.
This case underscores the rarity and complexity of synchronous twin malignancies in oncology. The management of such cases necessitates a personalised approach, integrating multimodal therapies and vigilant monitoring to optimise patient outcomes. Further research is needed to understand the underlying mechanisms driving synchronous malignancies and to develop tailored treatment strategies.







