Meeting Summary
This article captures key research highlights and new clinical evidence on chronic lymphocytic leukaemia (CLL) presented at the European Hematology Association (EHA) 2025, with a focus on first-line disease management.
Navigating Treatment Selection In CLL
Over the past decade, therapeutic advances in CLL have led to a seismic shift in the treatment landscape. A vast range of novel agents and different combination regimens are now available for the treatment of CLL, each with unique clinical profiles and offering the choice between continuous and fixed-duration (FD) therapy. Several sessions at EHA 2025 were focused on helping clinicians navigate this increasingly complex therapeutic landscape in CLL, using patient and disease-specific factors, plus the latest clinical and real-world evidence to guide treatment decision-making.
Some of the latest strategies and evidence-based approaches to managing CLL were discussed by experts in the BeOne-sponsored satellite symposium chaired by Clemens Wendtner, Professor and Medical Director of Munich Clinic Schwabing in Germany. Most patients with previously untreated CLL will have multiple viable treatment options, encompassing chemoimmunotherapy (CIT) and targeted therapies such as covalent Bruton’s tyrosine kinase inhibitors (BTKi) and B cell lymphoma 2 (BCL2) inhibitors. Treatment strategies can be broadly categorised into FD therapies and continuous BTKi-based regimens. The process of selecting treatment for first-line CLL is multidimensional and must consider numerous variables, including patient profile and preferences, disease- and treatment-related factors, treatment sequencing and resistance, and cost.
Predictive Biomarkers
In terms of disease biology, Ig heavy chain variable (IGHV) status, del(17p)/TP53 mutations, and complex karyotype are key predictive biomarkers. However, to date, there have been no dedicated clinical trials of these prognostic biomarkers, meaning that only post hoc analyses are available to guide treatment decision-making (Figure 1).1-8
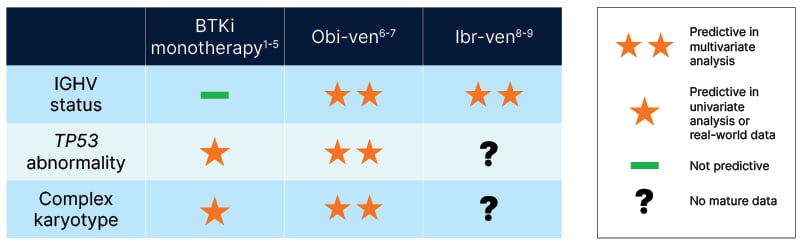
Figure 1: Predictive biomarkers in chronic lymphocytic leukaemia.1-8
BTKi: Bruton’s tyrosine kinase inhibitor; Ibr-Ven: ibrutinib/venetoclax; IGHV: Ig heavy chain variable; Ven-Obi: venetoclax-obinutuzumab.
The 2025 French Innovative Leukaemia Organisation (FILO) first-line treatment algorithm positions continuous BTKis before venetoclax-obinutuzumab (Ven-Obi) for patients with TP53 mutation and/or complex karyotype based on poorer outcomes with the latter in the CLL14 study.6,9 In contrast, the next-generation BTKi, zanubrutinib, has shown consistent efficacy across low- and high-risk CLL, while maintaining a favourable safety profile.1,10,11 Estimated 5-year progression-free survival (PFS) rates in the pivotal SEQUOIA study were 76% and 72% in del(17p) negative versus positive zanubrutinib-treated patients, respectively.1
Looking at the impact of karyotype, increasing complexity has been shown to be an independent predictor of inferior PFS and overall survival (OS) with ibrutinib in retrospective analysis.12 However, in the pooled venetoclax arms of the GAIA/CLL13 study and the SEQUOIA trial of zanubrutinib, ≥3 cytogenetic abnormalities (CA) did not significantly influence PFS versus ≤2 CAs (although ≥5 CAs was found to be an independent prognostic factor for inferior PFS with venetoclax).13,14
IGHV status can be another key factor influencing treatment choice in front-line CLL. The latest FILO guidelines position Ven-Obi as the first choice for mutated IGHV (mIGHV) and recommend FD targeted therapy with either Ven-Obi or ibrutinib/venetoclax (Ibr-Ven), or continuous BTKi for unmutated IGHV (uIGHV).9 In the SEQUOIA study, no significant difference was seen in PFS for mIGHV versus uIGHV patients treated with zanubrutinib, and similar PFS outcomes independent of IGHV status have also been reported for ibrutinib and acalabrutinib.1-3 In contrast, 5-year follow up of the GLOW and CAPTIVATE trials revealed shorter PFS in patients with uIGHV treated with Ibr-Ven as compared to mIGHV patients.7,8,15
Beyond Biomarkers
Beyond biomarkers, bulky disease and patient fitness can also help to guide front-line treatment selection. Bulky disease affected PFS outcomes with Ven-Obi in the CLL14 study, while consistent PFS benefit versus CIT was retained with zanubrutinib and Ibr-Ven, with or without ≥5 cm lymph node size, in the SEQUOIA and GLOW trials, respectively.1,6,15 Fitness also had no impact on PFS in patients treated with Ven-Obi in pooled analysis of the CLL13/14 studies, while Ibr-Ven was better tolerated by fit versus unfit patients in cross-trial comparison.15-17
Patient profile, comorbidities, and risk factors must also be considered during treatment decision-making in CLL. Based on outcomes from head-to-head studies, the second-generation BTKis acalabrutinib and zanubrutinib are generally recommended over ibrutinib for patients with CV risk.18,19 In the ALPINE trial comparing zanubrutinib to ibrutinib, atrial fibrillation/flutter occurred in 7.1% versus 17% of patients, while the rate of fatal cardiac adverse events was 0% versus 2%, respectively.20,21 A recent Bayesian network meta-analysis to assess the relative safety profile of first-line targeted therapies in patients with CLL with advanced age and/or comorbidities found that monotherapy was better tolerated than combinations, next-generation BTKis had the most favourable safety profile, and zanubrutinib was associated with the lowest risk of adverse events leading to discontinuation.22
Another key challenge facing clinicians involved in modern-day CLL management is the choice between continuous and FD therapies. This involves balancing factors such as safety and tolerability against efficacy and disease control, as well as practicality and convenience. Continuous therapies such as BTKis deliver sustained disease control in low- and high-risk disease, have a generally favourable safety profile suitable for use in elderly, unfit, or frail patients, and also benefit from convenient administration and monitoring.1-3,10,22 FD regimens, on the other hand, deliver more intense initial therapy with the aim of affording patients a treatment-free interval without continuous medication. This approach may be preferable for fitter, younger patients better able to tolerate higher toxicity, but comes with logistically more complex administration and monitoring schedules.6,8,15
Treatment After First Line
Looking beyond the front-line setting, treatment at first symptomatic relapse in CLL is dependent on both prior treatment sequencing and the type of relapse. In patients with relapsed/refractory disease (R/R) post-CIT or after continuous BTKi, FILO preference is for venetoclax-rituximab (Ven-R) based on results of the MURANO study, which showed a median PFS of 53.6 months for Ven-R versus 17.0 months for bendamustine-rituximab (BR).9,23 Where BTKis are recommended in the R/R setting, preference is for second-generation agents over ibrutinib based on head-to-head study results. In the ALPINE trial, zanubrutinib showed sustained PFS benefit over ibrutinib that was consistent across sensitivity analyses (36-month PFS rate: 64.5% versus 54.4%, respectively),20 while acalabrutinib proved non-inferior to ibrutinib for PFS in the ELEVATE-RR study, but with better CV tolerability.24
New Directions in CLL Therapy
New avenues under active exploration in CLL were discussed in an expert-led educational session, chaired by Martina Seiffert, Group Leader at the German Cancer Research Center. CAR-T cell therapy, notably lisocabtagene maraleucel (liso-cel), has demonstrated good long-term remissions in R/R CLL, but is not yet approved for this indication in Europe.25 Efforts to improve the efficacy of CAR-T cell therapy for CLL include: combination therapy with BTKis (which may enhance T cell performance and reduce exhaustion); use at earlier lines of therapy when the burden of disease is less; and exploration of novel targets like the B cell activating factor receptor. In the TRANSCEND-CLL 004 study, liso-cel plus ibrutinib produced higher complete remission rates compared to monotherapy (~20% versus 45%), with median duration of response and PFS not yet reached for patients in complete remission.26 Initial safety signals also revealed lower Grade 3 cytokine release syndrome and neurotoxicity with combination therapy, potentially due to ibrutinib’s anti-inflammatory effects.26 Currently, CAR-T cells are a very personalised therapy that take resources and money to produce but, in the future, ‘off the shelf’ options may become available. A ground-breaking trial (open to patients with CLL) is also ongoing, evaluating in vivo CAR-T cell therapy where the vector is administered directly to patients, allowing cancer-targeting T cells to be manufactured inside their own body.27
Long-term disease control is now achievable in CLL, with patients treated with continuous BTKis showing similar OS to an age-matched population.28 FD strategies have also been added to the armoury that aim to improve patient quality of life and reduce the risk of clonal evolution by offering protracted treatment-free intervals. However, a definitive disease cure remains elusive. New avenues under exploration in CLL include the use of MRD-guided strategies to determine optimal duration and number of therapies in a personalised approach to treatment. For example, the multicentre Phase II BOVen study of zanubrutinib with obinutuzumab and venetoclax used speed of MRD (MRD4+) to tailor therapy duration. This study met its primary endpoint, with 89% of previously untreated patients with CLL reaching undetectable MRD (uMRD) in both blood and bone marrow, despite median treatment of only 10 months.29
In terms of new drug development, more potent options targeting known disease pathways in CLL may help to transform long-term disease control into cure. The second-generation BCL2 inhibitor sonrotoclax, in combination with zanubrutinib, achieved 96% ORR and high uMRD rates in the ongoing global Phase I/Ib study (BGB-11417-101) in R/R CLL, and recruitment is now underway for the Phase III CELESTIAL trial.30,31 Another BCL2 inhibitor, lisaftoclax, has also shown efficacy in combination with acalabrutinib in patients progressed on venetoclax, using an accelerated daily ramp-up dosing schedule. A global Phase III registrational study (GLORA) is currently recruiting.32
BTK degraders aim to overcome treatment-emergent BTKi resistance mutations which can compromise efficacy.33 The BTK degrader BGB-16673 is a bivalent small molecule that binds specifically to BTK and the E3 ligase, marking it for destruction by the proteasome.34 At the 200 mg dose, BGB-16673 demonstrated an ORR of 94% in the ongoing open-label Phase I/II CaDAnCE-101 study in patients with double/triple refractory CLL. In all 49 response-evaluable patients, the ORR was 78% and the CR/CRi with incomplete hematologic recovery rate was 4%.35 Another BTK degrader, NX-5948, is at a slightly earlier stage of clinical development.33
Bridging the gap between malignant B and T cells in CLL, epcoritamab is a bispecific CD20-directed CD3 T cell engager that has shown early evidence of single-agent efficacy.36 Finally, inhibitors targeting the ERK/MEK pathway are under investigation as potential future therapeutic candidates for CLL.37
Insights From New Data in Front-Line CLL
A plethora of new clinical data and real-world evidence on front-line CLL management was presented at EHA 2025, providing further insights that will help to shape patient care moving forward.
Clinical Trials
The sustained efficacy of zanubrutinib in treatment-naïve, higher-risk patients with del(17p) was confirmed in 5-year follow-up data from the SEQUOIA trial, with patients continuing to demonstrate PFS benefits consistent with the randomised cohort of patients without del(17p) (Figure 2). A total of 111 treatment-naïve patients with CLL and del(17p) (median age: 71 years), were treated with zanubrutinib monotherapy in arm C of SEQUOIA. At median follow-up of 65.8 months, median PFS and OS were not reached. The estimated 60-month PFS and OS rates were 72.2% and 85.1%, respectively, with an ORR of 97.3%. No new safety signals were identified with longer-term follow-up, and zanubrutinib treatment remained ongoing in 62% of del(17p) carriers.38
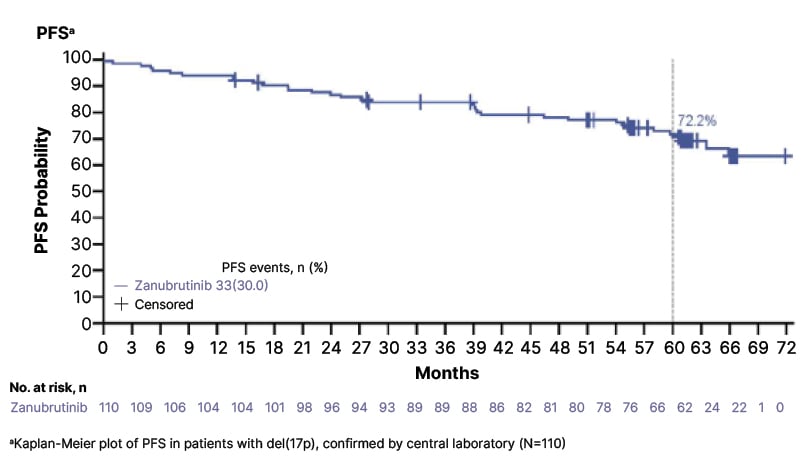
Figure 2: First-line zanubrutinib maintained progression-free survival benefits in patients with del(17p) over a 5-year follow-up in the SEQUOIA trial.
PFS: progression-free survival.
In the R/R setting, the randomised Phase III ALPINE study was the first trial to demonstrate PFS superiority in a global head-to-head comparison of BTKis, establishing the superiority of zanubrutinib over ibrutinib for both PFS and ORR.39 Final efficacy results from this trial at a 3.5 year follow-up using independent review committee-assessed responses were reported at EHA 2025. PFS rates at 36 months were 67.4% with zanubrutinib and 56.3% with ibrutinib, with PFS benefits in zanubrutinib-treated patients observed across major subgroups, including carriers of the del(17p)/TP53 mutation.39
The Role of MRD
A key theme at this year’s EHA was the current and future role of MRD, in particular its utility in informing decisions about stopping or restarting treatment. The ongoing Phase III FLAIR trial has become the first to confirm that Ibr-Ven with MRD-guided duration of treatment is superior to both continuous ibrutinib monotherapy and fludarabine, cyclophosphamide, and rituximab (FCR) for previously untreated CLL. Ibr-Ven significantly improved time to uMRD, PFS, and OS rates at 5-year follow-up. This trial supports the role of MRD in personalising treatment duration with BTKi-based combination therapy for first-line CLL in order to optimise patient outcomes.40
The relationship between MRD status and PFS was also evaluated in patients with CLL treated with first-line FD acalabrutinib-venetoclax combinations versus CIT in the ongoing AMPLIFY Phase III trial. The triple combination of acalabrutinib-venetoclax-obinuzumab (AVO) achieved the highest uMRD rates regardless of methodology or IGHV status and demonstrated uMRD durability up to 36 months after end of treatment (EOT). For both the acalabrutinib-venetoclax and AVO arms, achieving uMRD by EOT was associated with a significantly lower risk of disease progression or death versus FCR/bendamustine-rituximab for patients with uIGHV, with a similar trend seen among patients with mIGHV.41
The CAPTIVATE trial of first-line Ibr-Ven included FD and MRD-guided randomisation cohorts. In a final analysis of this trial, presented at EHA 2025, Ibr-Ven continued to provide durable PFS and OS with long-term follow-up, achieving 5.5-year PFS and OS rates of 66% and 97%, respectively. At EOT, 69% of patients were MRD negative in the peripheral blood. Around one-third (n=64) of patients experienced disease progression after completion of FD Ibr-Ven, with ibrutinib-based retreatment providing durable responses in patients needing subsequent therapy.42
Indirect Analyses
In the absence of prospective head-to-head trials investigating different BTKi and BCL2 FD treatment strategies, indirect comparisons afford a useful means of assessing comparative efficacy. However, these results should always be interpreted with some degree of caution given the limitations of indirect modelling methodology and potential sources of bias.
Indirect comparison of the AMPLIFY and CAPTIVATE trials has suggested that FD Ibr-Ven may offer a PFS advantage over acalabrutinib-venetoclax (Acala-Ven) in treatment-naïve, fit patients with CLL and without TP53 aberrations. This comparative analysis, which included 450 patients, found a 3-year restricted mean survival time difference of 2.7 months in favour of Ibr-Ven when high-risk patients were excluded.43
Similar findings were obtained in a cross-study comparison which compared pooled data from the CAPTIVATE FD cohort and Ibr-Ven arm of GLOW to the Acala-Ven arm of AMPLIFY. This analysis found that patients treated with Ibr-Ven achieved statistically significantly improved PFS outcomes and were significantly more likely to achieve uMRD at EOT+3 compared to patients treated with Acala-Ven.44
FD Acala-Ven also appeared inferior to continuous zanubrutinib treatment in a matching adjusted indirect comparison of the SEQUOIA and AMPLIFY trials. PFS was significantly superior for patients treated with zanubrutinib and 36-month PFS rates for zanubrutinib versus Acala-Ven were 88.5% and 76.5%, respectively. Collectively, these results suggest a significant PFS advantage of continuous zanubrutinib therapy over FD Acala-Ven in the first-line treatment of CLL.45
Real-World Evidence
Next-generation BTKi monotherapy is now the standard of care for treatment-naïve CLL, and recommended in guidelines.9 Real-world evidence is helping to reinforce the efficacy and safety results from BTKi clinical trials and establish optimised strategies for use of these agents in everyday clinical practice.
Results from a retrospective observational study of 2,515 patients from the USA Flatiron Health database treated with BTKi as their first-line monotherapy were presented at EHA 2025. First-line ibrutinib use was shown to decrease over time, with zanubrutinib being most commonly used by 2024 (49% versus 44% acalabrutinib, and 7% ibrutinib). Similarly, more patients treated with zanubrutinib in the real-world setting had a del(17p)/TP53 mutation: 16% versus 12% acalabrutinib, and 11% ibrutinib. In terms of efficacy, patients on zanubrutinib had a significantly longer real-world time to next treatment or death, time to treatment discontinuation or death, and overall survival compared to those on ibrutinib. Patients on zanubrutinib also showed longer trends compared with those on acalabrutinib (Table 1).46 A further real-world comparative effectiveness analysis conducted in USA community oncology practices looked at the likelihood of patients with CLL remaining on their initial first-line BTKi treatment and requiring subsequent therapy. Patients who received zanubrutinib were significantly more likely to remain on treatment compared with those who received acalabrutinib, and less likely to require the next line of therapy. At 2 years, there was a 53% probability of ongoing treatment with acalabrutinib compared to 76% with zanubrutinib, and 67% versus 72% probabilities, respectively, of not advancing to the next line of treatment.47
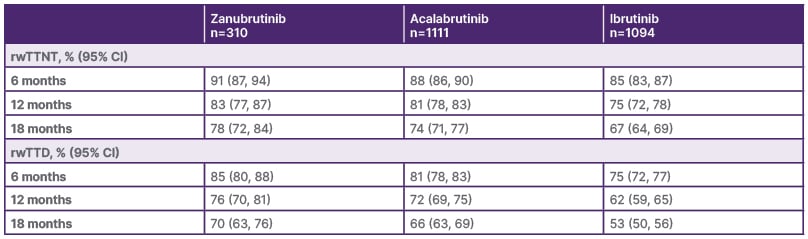
Table 1: Landmark probabilities of key real-world outcomes for patients on Bruton’s tyrosine kinase inhibitors.
rwTTD: real-world time to treatment discontinuation or death; rwTTNT: real-world time to next treatment or death.
Safety is also a key consideration in the real-world setting, particularly given the association between BTKis and CV events. A real-world study of 837 patients who received first-line BTKi captured data on new-onset of worsening hypertension over 12-months follow-up. Rates of new-onset hypertension were lower with zanubrutinib (13.9%) and acalabrutinib (12.4%) compared to ibrutinib (18.0%), with similar trends also observed for worsening hypertension.48
Conclusion
Several presentations at this year’s EHA Congress were aimed at helping clinicians map the evolving therapeutic landscape in CLL and make treatment decisions that enhance patient outcomes. Notable highlights in terms of new clinical evidence included updates from the ALPINE and SEQUOIA trials of zanubrutinib, and ground-breaking data from the FLAIR study showing the successful application of MRD-guided duration of treatment. Important evidence from real-world studies and indirect analyses were also presented, helping to further define optimal treatment strategies in CLL management.







