Co-chairs: Edoardo Campodonico,1 Annalisa Ruggeri2
Speaker: Sridhar Chaganti3
1. Innovative Immunotherapies, Immunology, Transplantation and Infectious Diseases, San Raffaele Hospital, Milan, Italy
2. Hematology and Bone Marrow Transplant Unit, San Raffaele Scientific Institute, Milan, Italy
3. University Hospital Birmingham, UK
Disclosure: Chaganti has received honoraria for advisory boards/consultancy from Kite-Gilead, Roche, Abbvie, Pierre Fabre, BMS-Celgene, Amgen, Sobi, and Autolus; speaker fees from Takeda, Kite-Gilead, Incyte, Pierre Fabre, and Roche; and meeting attendance support from Takeda, Kite-Gilead, Abbvie, and Pierre Fabre.
Disclaimer: The ALLELE study was sponsored by ATARA Biotherapeutics. Prescribing information and adverse events reporting for Ebvallo (tabelecleucel) for UK HCPs can be found here. For those outside the UK please refer to your local country of practice.AE reporting information can be found at the bottom of this article.
Support: The publication of this article was supported by Pierre Fabre.
Citation: EMJ Hematol. 2025;13[Suppl 2]:17-21. https://doi.org/10.33590/emjhematol/ANPO6479
Keywords: Epstein-Barr virus (EBV), haematopoietic stem cell transplantation, lym-phoproliferative diseases, organ transplantation, post-transplant complica-tions, post-transplant lymphoproliferative disease (PTLD), tabelecleucel.
![]()
Presentation Summary
This presentation was part of the ‘CAR-T and other cell therapies’ oral presentation session of the EBMT 51st Annual Meeting, held in Florence, Italy on 2nd April. Sridhar Chaganti, Consultant Haemato-oncologist at University Hospital Birmingham, UK, presented updated results from the ongoing Phase III ALLELE trial evaluating the safety and efficacy of tabelecleucel, an off-the-shelf allogeneic T cell immunotherapy, in relapsed/refractory Epstein-Barr virus (EBV)-positive post-transplant lymphoproliferative disease (PTLD). In this latest analysis in a total of 75 patients, the overall response rate (ORR) in recipients of hematopoietic cell transplant (HCT) or solid organ transplants (SOT) was 50.7%, with median response duration of 23 months and a 12-month overall survival (OS) rate of 78.7% in responders. Tabelecleucel was well tolerated, with no cases of tabelecleucel-related graft-versus-host disease or organ rejection reported.
The Phase III ALLELE Study of Tabelecleucel in Relapsed/Refractory Epstein-Barr Virus + Post-Transplant Lymphoproliferative Disease
PTLDs are a serious complication in patients who have received allogeneic HCT or SOT. They are frequently caused by the presence of EBV that resides in B lymphocytes and can cause uncontrolled B cell proliferation.1 Current treatments for EBV+ PTLD include a reduction in immunosuppression (RIS) and treatment with anti-CD20 antibody (rituximab) with or without chemotherapy.1 However, the response rates to these treatments are variable, and in patients with EBV+ PTLD who do not respond, the median OS rates are 0.7 months for those receiving HCT and 4.1 months after SOT.1 There is an urgent unmet need for new treatment options for this patient population.
Tabelecleucel is an off-the-shelf, non-genetically modified, allogeneic, EBV-specific cytotoxic T cell immunotherapy that targets and eliminates EBV-infected cells in an HLA-restricted manner and has been investigated for multiple EBV-associated malignancies.2 Tabelecleucel is manufactured from unrelated healthy donor T cells, enriched to recognise EBV antigens, characterised by their HLA restriction and cryo-preserved in a biobank. Each product includes a polyclonal population of highly specific anti-EBV T cells capable of recognizing different EBV-derived peptides: HLA combinations presented on the surface of the infected tumour target cells (HLA restriction). For each patient, a tabelecleucel lot is selected based on an appropriate HLA restriction and matching to optimise anti-tumour activity and recipient compatibility, with the potential to switch lots up to four times if no response is obtained.
The ALLELE study is an ongoing, global, Phase III multicentre, open-label trial investigating the efficacy and safety of tabelecleucel after failure of rituximab ± chemotherapy in patients with EBV+ PTLD following HCT or SOT. Previously reported results from 43 patients (14 HCT; 29 SOT) suggest that tabeleleucel was well tolerated and showed an objective response rate of 50% in the HCT group and 52% in the SOT group.3 Based on these data, tabelecleucel was approved in Europe in 2022.4
Updated Results from the ALLELE Trial
During this oral presentation, Chaganti presented the updated results on efficacy and safety from a larger cohort of 75 EBV+ PTLD patients who have now been treated, 26 post-HCT and 49 post-SOT. They outlined the key eligibility criteria for patients recruited to ALLELE who had a biopsy-proven EBV+ PTLD, had failed treatment with rituximab or rituximab and chemotherapy, and had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤3. The primary endpoint of the trial was ORR, and key secondary endpoints were time to response (TTR) and time to best response, overall survival (OS) in responders versus non-responders, progression-free survival (PFS) in responders, and rates of allograft loss/rejection episodes (SOT).
Tabelecleucel was administered in cycles that lasted 5 weeks each, during which patients received intravenous administration of the product at a dose of 2×106 T cells/kg patient body weight on Days 1, 8, and 15, with 1-hour monitoring post-injection. Chaganti explained that there was no requirement for pre-medication or lymphodepletion, and most patients received tabalecleucel in the outpatient setting.
Response assessments were performed in Week 5. Patients were allowed to continue with further cycles, and depending on their response, had the option of switching to a different HLA restriction product lot.
Chaganti presented the baseline characteristics of the 75 patients who had been enrolled into the ALLELE study at the cut-off date. Patients’ median age was 44 years (range 2–81 years). Overall, 26.5% of patients had an ECOG score ≥2 and a majority had extranodal disease at screening (74.7%). Almost all (92.6%) had an intermediate- or high-risk PTLD prognostic score. Most (69.3%) had PTLD of diffuse large B cell lymphoma morphology.
The median number of previous lines of therapy was one (range 1–5). Of 26 patients in the HCT cohort, all of them had failed prior rituximab treatment and four patients had previously received chemotherapy. There were 49 patients in the SOT cohort, of which 79.6% had already received rituximab and 57.1% had received rituximab and chemotherapy. SOT types included kidney, heart, lung, liver, and multivisceral.
Patients received a median of two cycles of tabelecleucel and had a median treatment duration of 1.9 months. A majority of infusions were carried out in the outpatient setting, with 65.3% of patients receiving one product lot, 30.7% receiving two product lots with different HLA restrictions, and 4% receiving three product lots.
Chaganti went on to present the updated primary endpoint of ORR, which was 50.7% across both HCT and SOT cohorts (38 of 75 patients; Figure 1). Of these 38 patients, 21 (28%) had a complete response and 17 (22.7%) had a partial response (Figure 1). The median duration of response was 23 months and the median time to response was 1.1 months.
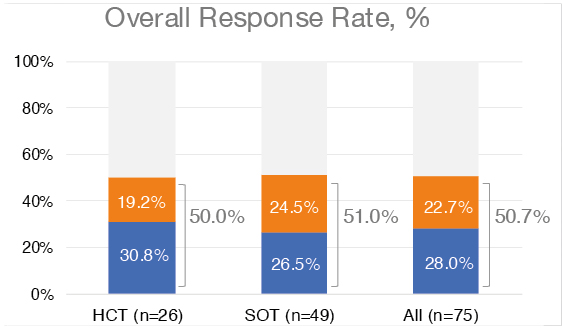
Figure 1: Overall response rate to tabelecleucel assessed per Lugano classification with Lymphoma Response to Immunomodulatory Therapy Criteria (LYRIC) modification by independent oncologic response adjudication.
Data cutoff date: 9th October 2023.
CR: complete response; HCT hematopoietic cell transplant; PR: partial response; SOT: solid organ transplant.
Moving on to secondary endpoints, the proportion of responders with PFS at 12 months was 71.9%, and the median PFS among responders of nearly 2 years (23.9 months; Figure 2).
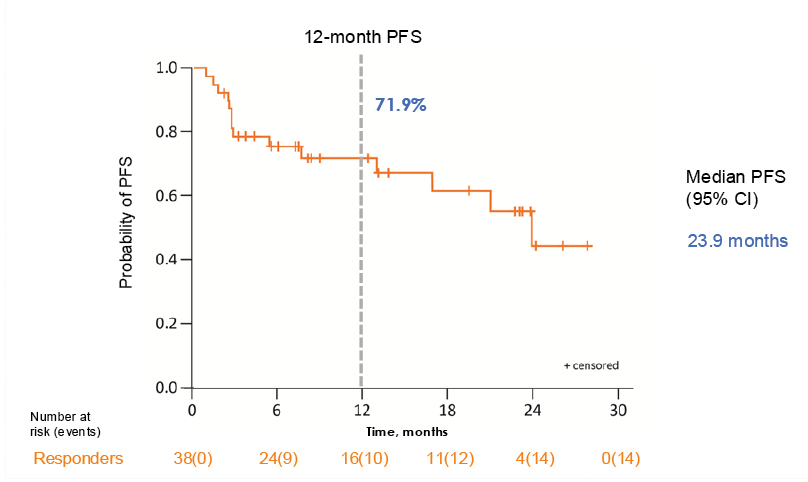
Figure 2: 12-month progression-free survival among responders.
Data cutoff date: 9th October 2023. Median PFS was estimated by the Kaplan–Meier method.
PFS: progression-free survival.
At a median follow-up of 9 months, the 12-month OS was 55.7% for all patients treated and 78.7% for responders to tabelecleucel compared to 28.2% in non-responders (Figure 3). The median OS for all patients was 18.4 months, but this was not evaluable for responders.
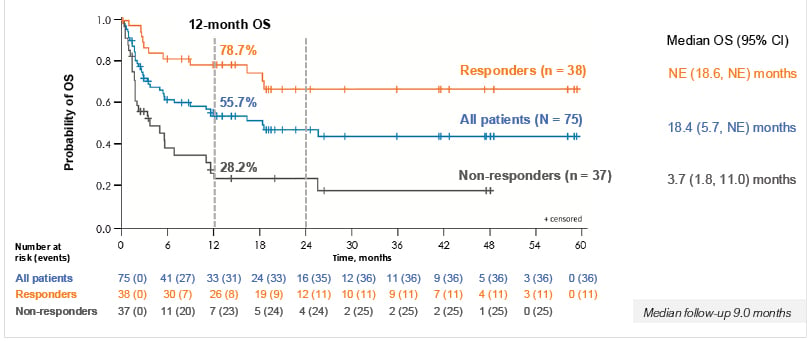
Figure 3: Responders to tabelecleucel had improved 1-year overall survival compared to non-responders.
Data cutoff date: 9th October 2023. OS was estimated by the Kaplan–Meier method. Median follow-up was 9 months.
NE: not estimable; OS overall survival.
Treatment-emergent serious adverse events were not infrequent in this population, but treatment-related serious adverse events were uncommon. There were no reports of infusion-related reactions, immune effector cell-associated neurotoxicity syndrome, tumour flare reaction, cytokine release syndrome immunogenicity, or infection disease transmission. There were three reports of organ rejection in the SOT group, but these were not considered to be related to tabelecleucel.
Post-Presentation Question and Answer
Following the presentation, co-chair Campodonico said the study provided ‘food for thought’ for other EBV-positive diseases. They asked whether there was a difference in ORR between HCT and SOT recipients. Chaganti confirmed that they did not find any difference. The ORR was 50% for patients who received HCT and 51% for patients who received SOT (Figure 1).
Conclusion
In their conclusion, Chaganti summarised that these updated data from the ALLELE study confirm the previously reported benefit of tabelecleucel in relapsed/refractory EBV+ PTLD. They added that tabelecleucel had a promising ORR and OS in this difficult-to-treat patient population and responses were durable, with a median response duration of 23 months. The treatment appeared to be well tolerated, with most treatment-emergent serious adverse events unrelated to tabelecleucel.
| Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should be reported to Pierre Fabre on 0800 0855292, [email protected] or https://www.pierre-fabre.com/en/vigilance-form. |







