Abstract
Secondary forms of metabolic dysfunction-associated steatotic liver disease (MASLD) have different pathogeneses, outcomes, and specific treatment approaches. The aim of this narrative review is to discuss the principal forms of MASLD secondary to endocrine disorders. MASLD is associated with hypothyroidism both in adults and in children. Impaired intrahepatic thyroid hormonce receptor β signalling contributes to the progression of metabolic dysfunction-associated steatohepatitis and explains why resmetirom, a liver-specific thyromimetic, improves lipid profile and liver histology in this condition. Thyroid-stimulating hormone testing should be performed in all patients with MASLD. Women with the classic hyperandrogenic polycystic ovary syndrome (PCOS) phenotype are strongly at risk of MASLD, suggesting that hyperandrogenism determines visceral adiposity, metabolic dysfunction, and progressive MASLD in this patient population. All women with PCOS should therefore undergo MASLD screening. The risk of MASLD is significantly increased among growth hormone deficiency (GHD) subjects versus matched controls without GHD. This is due to the roles of growth hormone (GH) and insulin-like growth factor 1, which act on various hepatic cell types to mitigate the progression of steatosis and liver fibrosis. In adults, GHD presents with central obesity, sarcopenia, and osteopenia. MASLD rapidly develops among subjects with hypothalamic-pituitary diseases and ‘hypothalamic obesity’ owing to impaired GH/ insulin-like growth factor axis, hypogonadotropic hypogonadism, and hypothyroidism. Medical history of any conditions predisposing to panhypopituitarism may offer clues to identify panhypopituitarism-related MASLD, which may also be suspected in subjects with ‘cryptogenic’ cirrhosis and hypernatremic hyperosmolality. These MASLD forms secondary to endocrine disorders carry important implications for further research and clinical practice. Endocrine aspects of MASLD may disclose novel therapeutic pathways. A high index of suspicion is requested in clinical practice to triage subjects with MASLD secondary to endocrine disorders.
Key Points
1. The evolving nomenclature of metabolic dysfunction-associated steatotic liver disease (MASLD) still requires conceptual and nosographic refinement of disease forms secondary to specific endocrine disorders. These endocrine MASLD forms have distinct manifestations, pathogenesis, and treatment approaches.
2. Here we describe MASLD forms secondary to hypothyroidism, polycystic ovary syndrome, growth hormone deficiency, and panhypopituitarism. For each of these conditions, theevidence, mechanisms, and diagnosis are illustrated.
3. Clinicians should be aware that hypothyroidism, polycystic ovary syndrome, growth hormone deficiency, and panhypopituitarism are risk factors for the development and progression of MASLD. Triaging these MASLD forms secondary to endocrine disorders is key for early diagnosis and specific treatment.
BACKGROUND
Defined as the accumulation of triglycerides within hepatocytes exceeding 5% of liver weight, non-alcoholic fatty liver disease (NAFLD) encompasses a gamut of disorders from simple steatosis to non-alcoholic steatohepatitis (NASH), with or without fibrosis, cirrhosis, and hepatocellular carcinoma. ‘Primary’ NAFLD is typically associated with either the full-blown metabolic syndrome (MetS) or its individual constituents that represent manifestations of insulin resistance and often arise in association with visceral obesity.
There is a strong rationale for distinguishing primary NAFLD from secondary forms of steatotic liver disease (SLD), which have distinctly different pathogenesis, outcomes, and specific treatment approaches.1-3 However, the spectrum of SLD forms secondary to endocrinopathies has evolved over time (Table 1).3-7
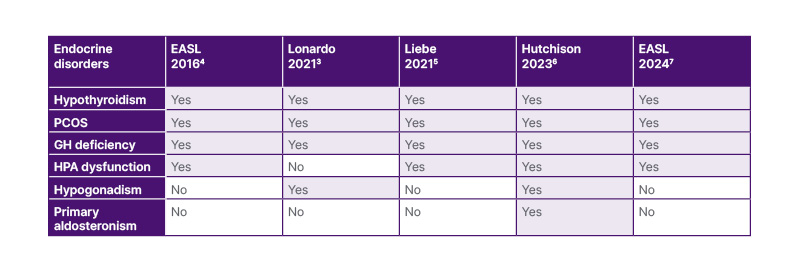
Table 1: The evolving nosography of steatotic liver disease forms secondary to selected endocrine disorders.
EASL: European Association for the Study of the Liver; GH: growth hormone; HPA: hypothalamic-pituitary axis; PCOS: polycystic ovary syndrome.
In 2002, Angulo listed four categories of what he called “secondary NAFLD”, referring to SLD forms not associated with the MetS: nutritional, drug-induced, owing to rare genetic and metabolic causes, and ‘others’, which includes inflammatory bowel disease, infection with HIV, and hepatotoxins.1 In 2012, Kneeman et al.2 also mentioned hepatitis C virus infection among the secondary forms of SLD. However, neither Angulo nor Kneeman described any NAFLD forms secondary to endocrine disorders.
In 2006 and 2009, the pathogenic role of endocrine pathways in NAFLD and NASH, respectively, was clearly described.8,9 In 2010, the Italian guidelines on NAFLD suggested suspecting polycystic ovary syndrome (PCOS) in women in whom NAFLD was associated with altered menses and hirsutism.10 The 2016 European Association for the Study of the Liver (EASL) guidelines suggested investigating thyroid diseases, and PCOS as a part of the ‘extended diagnostic protocol’ of suspected NAFLD, to be implemented based on a priori probability of disease.6 Studies published in 2021 further contributed to encoding secondary SLD forms.3,5
In 2023 ‘fatty liver’ was renamed as SLD, and NAFLD was replaced with MASLD.11 While NAFLD identifies SLD for what it is not, the MASLD definition is based on the positive recognition of the prominent role played by metabolic dysfunction in the development of SLD. SLD may be classified as either cryptogenic (with no clear cause) or secondary to specific aetiologies comprising drugs, monogenic disease, or miscellaneous forms such as hepatitis C virus, malnutrition, celiac disease, and HIV.11 However, endocrine disorders are not mentioned in this classification.11
The 2024 EASL MASLD guidelines clearly state that SLD may be secondary to some specific endocrinopathies, such as hypothyroidism, PCOS, growth hormone (GH) deficiency (GHD), and panhypopituitarism.7 In addition to these conditions, Hutchison et al.6 have also added hypogonadism and primary hyperaldosteronism aspotential contributors.
Here the authors discuss the principal endocrine disorders associated with secondary SLD/MASLD forms (Figure 1). For each endocrinopathy, they reappraise the evidence supporting the association with MASLD, the pathomechanisms involved, and finally pinpoint the specific diagnostic strategy to adopt.
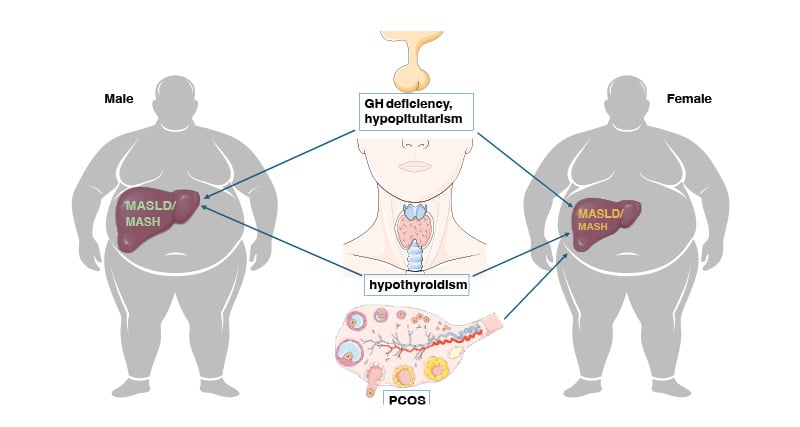
Figure 1: Metabolic dysfunction-associated steatotic liver disease/metabolic dysfunction-associated steatohepatitis; secondary to selected endocrine disorders: growth hormone deficiency, hypopituitarism, hypothyroidism, and polycystic ovary syndrome.
Overview of the best characterised among the so called MASLD forms secondary to endocrine disorders. The identification of these distinct MASLD forms requires a high index of suspicion and carries relevant diagnostic, prognostic, and therapeutic implications.
GH: growth hormone; MASH: metabolic dysfunction-associated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease; PCOS: polycystic ovary syndrome.
MASLD IN HYPOTHYROIDISM
The Evidence
Robust epidemiological evidence supports the association of hypothyroidism and MASLD both in adults and in children (Table 2).12-15
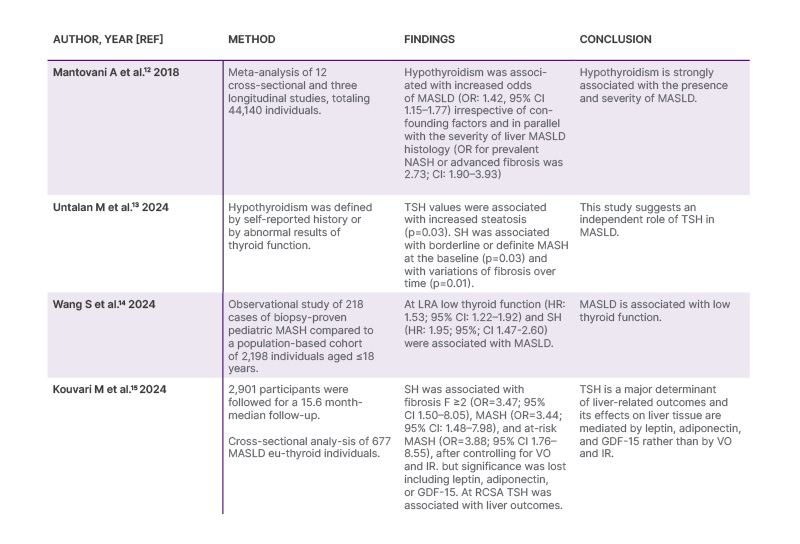
Table 2: Evidence for the association of hypothyroidism and metabolic dysfunction-associated steatotic.
GDF-15: growth differentiation factor-15; IR: insulin resistance; LRA: logistic regression analysis; MASH: metabolic dysfunction-associated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease: OR: odds ratio; RCSA: restricted cubic spline analysis; SH: subclinical hypothyroidism; TSH: thyroid stimulating hormone; VO: visceral obesity.
The Mechanisms
Euthyroid MASH individuals have a reduced ability to convert the prohormone thyroxine to the bioactive triiodothyronine form while also exhibiting increased conversion of prohormone thyroxine to the inactive reverse triiodothyronine metabolite.16 These changes connote local hypothyroidism owing to impaired intrahepatic TRβ signalling, which promotes MASH progression and explains why Resmetirom, a liver-specific thyromimetic, improved liver inflammation, fibrosis, and lipid profile in patients with MASH through ameliorated intrahepatic TRβ signalling.16
The Diagnosis
Primary stable hypothyroidism, resulting from thyroid failure, can occur because of chronic autoimmune thyroiditis or iatrogenic procedures; while transient hypothyroidism may result from silent thyroiditis.17 Therefore, careful medical history taking may offer important diagnostic clues in the individual patient. However, based on expert opinion, Thyroid-stimulating hormone (TSH) testing should be performed in all patients with MASLD to rule out MASLD forms secondary to hypothyroidism.18 To differentiate sub-clinical from ‘clinical’ (i.e. overt) hypothyroidism necessitating hormone replacement therapy, free thyroid hormone levels should be assessed whenever TSH is abnormal.
MASLD IN PCOS
The Evidence
A meta-analysis of 17 published studies totalling 2,734 patients with PCOS and 2,561 age- and BMI-matched controls19 found that patients with PCOS had a higher risk of prevalent MASLD (odds ratio [OR]: 2.54; 95% CI: 2.19–2.95). This study showed that, compared to PCOS women without hyperandrogenism, the classic hyperandrogenic PCOS phenotype had a higher prevalence of MASLD irrespective of confounders.19
PCOS is independently associated with more severe forms of MASH, including advanced liver fibrosis. Retrospective analysis of a multi-ethnic cohort of 102 women aged 18–45 years with biopsy-proven MASH20 found MASH in 76% of women with PCOS, compared to 66% of those without it. Age- and BMI- adjusted analysis showed that PCOS was associated with severe hepatocyte ballooning (OR: 3.4; 95% CI: 1.1– 0.6; p=0.03) and women with advanced fibrosis were, on average, 5 years younger than those without PCOS, indirectly suggesting that PCOS may accelerate the fibrotic progression of MASH.20
The Mechanisms
Various pathomechanisms, including the individual features of the MetS, account for the increased prevalence of MASLD and its accelerated histological progression among women with PCOS.18 Obesity-associated insulin resistance, by promoting intrahepatic steatogenesis, hyperandrogenism, and chronic anovulation, is a strong pathogenic nexus associating PCOS and MASLD.18
However, young women with PCOS exhibit a high risk of MASLD/MASH irrespective of obesity, suggesting that hyperandrogenism is the most likely causal factor determining the accumulation of visceral adiposity, metabolic dysfunction, and progressive MASLD in this patient population.21,22
The Diagnosis
Clinically, amenorrhea and hirsutism in a woman of fertile age should raise the suspicion of PCOS, even in the absence of visceral obesity,23 which justifies gynaecological referral for further assessment. PCOS is defined by the presence of at least two out of three factors: oligo-anovulation, clinical or biochemical hyperandrogenism, or ≥12 follicles in a single ovary or ovarian volume >10 cm3.24
All women with PCOS should undergo MASLD screening, including family history of liver disease, physical examination, clinical and endocrinological phenotyping, liver enzymes, MASLD biomarkers, and imaging techniques that quantify steatosis, steatohepatitis, and fibrosis.25 Liver biopsy should be reserved for selected cases who either prove non-responsive to first-line lifestyle changes or whenever competing causes of chronic liver disease are suspected other than PCOS.26
GROWTH HORMONE DEFICIENCY
The Evidence
Observational and interventional studies support the notion that GHD causes a distinct type of MASLD.
A pioneering study27 found that serum GH concentrations were significantly lower in 61 MASLD subjects compared to 104 controls (0.03 ng/mL versus 0.1 ng/mL, respectively, p<0.01) and that reduced GH levels independently predicted MASLD in male patients, suggesting the existence of a central neuroendocrine dysregulation.
Twenty-one years later,28 a meta-analysis of 10 published studies totalling 782 participants showed that the risk of MASLD was significantly higher in patients with GHD compared to matched controls without GHD (pooled OR: 4.27; 95% CI: 1.33–13.68%; p=0.015). The prevalence of MASH in subjects with GHD (18% versus 1.33%) was significantly higher than in the general population, supporting targeted strategies for the early diagnosis of MASLD/MASH in patients with GHD.28
A meta-analysis on the efficacy and safety of GH therapy in MASLD29 assessing data from three randomised controlled trials totalling 120 patients, found that 6 months of GH therapy in MASLD significantly reduced intrahepatic lipid content compared to placebo (p=0.04). Consistently, GH treatment was associated with significantly higher reduction of visceral adipose tissue area (p=0.03), significantly raised serum insulin-like growth factor-1 (IGF-1) (p<0.0001), significantly reduced high-sensitivity C-reactive protein (p=0.0006) and gamma-glutamyl transpeptidase (p=0.0008) without any significant changes in fasting glucose, triglyceridemia, and new-onset hypothyroidism.29 Additional studies are needed to assess the impact of GH therapy on liver fibrosis progression.
The Mechanisms
GH is a key player of growth in children and metabolic health in adults. GHD exhibits visceral obesity, metabolic dysfunction, sarcopenia, and osteopenia,30 which are potentially reversible with hormone replacement therapy,30 and are recognised risk factors for MASLD and fibrosing MASH.
Moreover, GH stimulates the liver to secrete circulating IGF-1, and GHD can progress to end-stage liver cirrhosis in some adults and children.31 Interestingly, GH and IGF-1 can act on hepatocytes, macrophages, and hepatic stellate cells to mitigate progression of steatosis and fibrosis.31
The Diagnosis
GHD in adults is commonly associated with a non-specific clinical presentation featuring central obesity, and decreased muscle and bone mass,31 which may raise the suspicion of GHD in selected cases. However, the link between GHD and MASLD is often overlooked and underrecognised,32 and no established recommendations specify when GHD should be investigated among patients with MASLD in clinical practice. While MASLD and MASH subjects frequently exhibit decreased levels of GH and IGF-1, the true clinical significance of these findings remains unclear, and further studies are needed to determine the most appropriate and cost-effective medical conduct to follow in this field.
Whenever specialistic assessment is requested for those referred to the hepatologist after documented diagnosis of GHD, it will be important to recognise MAFLD/MASH. Indeed, GH replacement therapy, combined with management of concurrent metabolic dysfunction, should be considered in these patients.31
Increased awareness of the GHD and MASLD association would facilitate early diagnosis of comorbid MASLD/MASH. Therefore, a multidisciplinary approach involving hepatology and endocrinology should become a standard of care for these patients.32
PANHYPOPITUITARISM
The Evidence
The first clinical evidence of ‘hypothalamic obesity’ developing in patients with hypothalamic-pituitary diseases dates back to 1840.33 This syndrome, featuring intense overfeeding, locomotor inactivity, altered carbohydrate metabolism, and massive hepatic steatosis, was experimentally characterised in mammals in 1944.33 In 2004, a study reported 21 patients who developed MASLD 3 years after being diagnosed with hypothalamic-pituitary dysfunction.34 Similar to the experimental studies in mammals, these patients experienced a rapid development of metabolic dysfunction and MASLD, which could not be attributed to any explanations other than the neuroendocrine dysfunction itself.34 Recent progress in metabolomics has clarified subtle pathomechanisms driving fibrosing MASLD in individuals with hypothalamic obesity.35
The Mechanisms
MASLD developing in the context of panhypopituitarism is multifactorial and involves an impaired GH/IGF axis, hypogonadotropic hypogonadism, and hypothyroidism.33 Each of these endocrinopathies contributes to MASLD development and its fibrotic progression through mechanisms such as lipid peroxidation, exacerbated oxidative stress, reduced glutathione, modification in cytochrome P450s, mitochondrial dysfunction, PPARA activation, altered metabolism of fatty acids and NADP, enhanced peroxisome β-oxidation and ω-oxidation, downregulation of the Janus kinase 2–signal transducer and activator of transcription 5B, and upregulation of mTOR signalling pathway.35,36
The Diagnosis
Detailed medical history capturing any genetic, neoplastic, traumatic, or inflammatory predisposing conditions, may represent clues to panhypopituitarism-related MASLD. This condition may also be suspected in subjects with ‘cryptogenic’ cirrhosis and hypernatremic hyperosmolality, which are positively associated with fasting hyperinsulinaemia,37 and should prompt endocrinological referral for early recognition and management of panhypopituitarism.
CONCLUSION
Ever-increasing awareness of the secondary MASLD forms calls for classifying ‘endocrine MASLD’ as a distinct disease pathotype for which replacement hormone therapy offers the opportunity of pathogenic treatment and, in principle, full MASLD reversal. Multidisciplinary approaches should routinely be offered to these patients. Additionally, endocrine MASLD forms may disclose novel therapeutic pathways, such as proven by resmetirom. Based on the considerations illustrated in the present study, a high index of suspicion is requested in clinical practice to triage subjects with MASLD/MASH secondary to endocrine disorders







