Interview Summary
Primary biliary cholangitis (PBC) is characterised by the presence of disease-specific autoantibodies and persistently elevated liver enzymes. The underlying hepatic lesions cause progressive bile duct destruction, resulting in cholestasis and associated symptoms. PBC is also associated with symptoms such as pruritus, fatigue, and sicca syndrome, which can significantly impact health-related quality of life (HR-QoL) and typically do not improve with first-line standard-of-care therapy (ursodeoxycholic acid [UDCA]).
EMJ interviewed three leading experts in PBC: Thomas Berg, Professor of Medicine, Head of Hepatology, and Deputy Director of Medicine II at Leipzig University Medical Centre, Germany; Michael Trauner, Professor of Medicine and Chair of Gastroenterology and Hepatology at the Medical University of Vienna, Austria; and Palak Trivedi, Clinician Scientist in the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre, and Associate Professor and Honorary Consultant Hepatologist at the Centre for Liver and Gastrointestinal Research at the University of Birmingham, UK. In this article, the experts discuss the unmet needs in PBC management, and the paradigm changes that they anticipate in the field with the recent approval of two peroxisome proliferator-activated receptor (PPAR) agonists: elafibranor and seladelpar.
Efficacy and safety data from the Phase III trials of these PPAR agonists are considered alongside post-hoc analyses and interim data from long-term extensions. The experts offer their perspectives on the future management of PBC and the changes they expect to see in practice guidelines, including the assessment of biochemical response to UDCA at an earlier stage, particularly in patients with high-risk features. Finally, outstanding questions in the understanding and management of PBC are raised, indicating areas for future research.
INTRODUCTION
Primary biliary cholangitis (PBC) is a rare autoimmune cholestatic liver disease associated with a risk of developing cirrhosis and portal hypertension, as well as symptoms such as pruritus and fatigue that have a significant impact on patients’ health-related quality of life (HR-QoL).1-4 Clinically, PBC is characterised by a persistent elevation of liver enzymes, including alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT), and the presence of specific autoantibodies, with hepatic lesions that lead to bile duct destruction and cholestasis.2
Both Berg and Trivedi emphasised that the global incidence and prevalence of PBC is increasing, particularly among men.5,6 This may be due, in part, to increased routine testing of liver function, resulting in more patients being diagnosed with a milder severity of disease.7
In Berg’s experience, patients with PBC tend to fall into one of three groups (Berg, Personal correspondence):
Group 1
Patients who are referred to a hepatologist with elevated liver enzymes and are subsequently diagnosed via a positive anti-mitochondrial antibody test. These patients have little-to-no symptoms and respond well to standard-of-care therapy, with a complete biochemical response. After many years, they may experience disease relapse requiring second-line treatment.
Group 2
Patients who present with more active disease, such as ALP ≥3 times the upper limit of normal (ULN). These patients rarely achieve complete remission with standard-of-care therapy, and second-line treatment is usually required soon after diagnosis.
Group 3
Patients who present with various degrees of biochemical disease activity but who experience disease symptoms such as pruritus, fatigue, sicca symptoms, and/or joint pain. These symptoms often fail to respond completely to standard-of-care therapy, and additional medication is usually required.
Berg, Trauner, and Trivedi discussed the current treatment options for PBC, and the paradigm changes that they anticipate in the field, particularly in respect to the recent approval of two peroxisome proliferator-activated receptor (PPAR) agonists, elafibranor and seladelpar.8,9
LIMITATIONS OF CURRENT MANAGEMENT OF PRIMARY BILIARY CHOLANGITIS
Current guidelines recommend first-line treatment with ursodeoxycholic acid (UDCA), followed by an assessment of biochemical response at 12 months.1,10,11 In patients with an inadequate biochemical response to UDCA, adjunct treatment with obeticholic acid (OCA) is recommended,1 and both Berg and Trivedi mentioned that that off-label treatments such as bezafibrate are often used. At the time of writing, OCA was also an off-label treatment in the EU, following revocation of conditional marketing authorisation by the European Commission in November 2024, citing that the benefits of OCA no longer outweigh its risks.12,13 This decision has faced criticism from some HCPs and patients.14-16 OCA has demonstrated long-term safety, with stable remission in approximately half of patients.17 However, OCA has also been associated with rare instances of liver decompensation and failure, and may worsen pruritus.17 Thus, efficacious and safe long-term treatments for PBC are needed.18
Management of Symptoms
Alongside treatment to slow liver disease progression in PBC, guidelines recommend that symptoms should be evaluated and actively managed, since symptom severity does not always correlate with disease stage and they typically do not improve with UDCA therapy.1,10,11
“People are surviving longer with PBC,”19 explained Trivedi, “but with that comes a greater need to treat symptoms.” Berg agreed that adequate treatment of symptoms may be the greatest unmet need among patients with PBC.2 “[Research has] mainly focussed on controlling disease activity through biochemical response,” he explained, “and less on patient reported outcomes.” However, pruritus and fatigue are common symptoms of PBC that impact HR-QoL, and some patients also experience joint pain, sicca symptoms, and cognitive dysfunction.10,20 Trauner added that the burden of symptoms and their impact on treatment adherence in PBC can be underestimated, emphasising that in his experience “patients are less willing to take a medication that has little impact on their symptoms” (Trauner, personal communication).
Management of Advanced, Decompensated Disease
The majority of therapeutic options for PBC are targeted at patients with well-compensated liver function.1,10,11 However, Trivedi emphasised that less has been done to address those with more advanced liver disease who require liver transplantation, and may have jaundice, cirrhosis, and clinically significant portal hypertension. “This group of patients has a clear unmet need,” he explained, “however, importantly, we do now have treatments that will reduce the number of patients who reach that stage”.
Assessing Biochemical Response to Treatment
Current guidelines recommend assessment for biochemical response after 12 months of UDCA therapy.1,10,11 Several published criteria, such as Rochester, Barcelona, Paris, Rotterdam, Toronto, UK PBC, and Globe scores, can be used to define a biochemical response, focussing on ALP and bilirubin values.21
Berg agreed that normalisation of ALP is critical to reduce disease progression.22,23 However, he emphasised the additional importance of normalising GGT, another marker of cholestasis,24 and markers of inflammation such as alanine transaminase (ALT), and aspartate transaminase (AST),10 particularly in patients who present with advanced fibrosis.
The recommendation to assess biochemical response at 12 months is likely to be amended in future guidelines, particularly with the addition of more second-line treatment options.25 “I think the historical paradigm of waiting 12 months is somewhat archaic,” said Trivedi, “and the movement towards assessing biochemical response at an earlier stage is welcomed.” At 6 months, clinicians can usually tell whether a patient is on a trajectory to achieving biochemical response or even normalising their ALP.25
While assessment at 12 months may be adequate for the majority of patients, intervening at an earlier stage is particularly important in patients with high-risk features, such as younger presenting age, and elevated liver enzymes and bilirubin.25 The damage to bile ducts in PBC with an aggressive disease course is irreversible,1 and there is a lack of biomarkers to identify which patients are at risk of bile duct loss.26 Berg and Trauner both stressed the importance of early monitoring of biochemical response and early intervention in patients with high-risk features, to avoid permanent liver damage.
The logic of waiting for 12 months to assess biochemical response is further challenged by the approval of two PPAR agonists as second-line treatment options for PBC.25
PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR AGONISTS
PPARs are nuclear receptors that play a key role in regulating the transcription of genes involved in inflammation, metabolic pathways, and carcinogenesis, making them potential targets for the treatment of cholestatic liver diseases, including PBC (Figure 1).27 There are three isotypes of PPARs: α, γ, and δ, each with specific tissue expression and target genes.27 In the liver, PPARs are involved in counteracting inflammation, fibrosis, and steatosis, and regulating bile acid metabolism.27 In PBC, fibrates (fibric acid derivatives) such as bezafibrate and fenofibrate have been used as lipid-lowering molecules for over 50 years, and have been investigated for use in PBC, though they are currently not approved by regulatory authorities for this indication.27
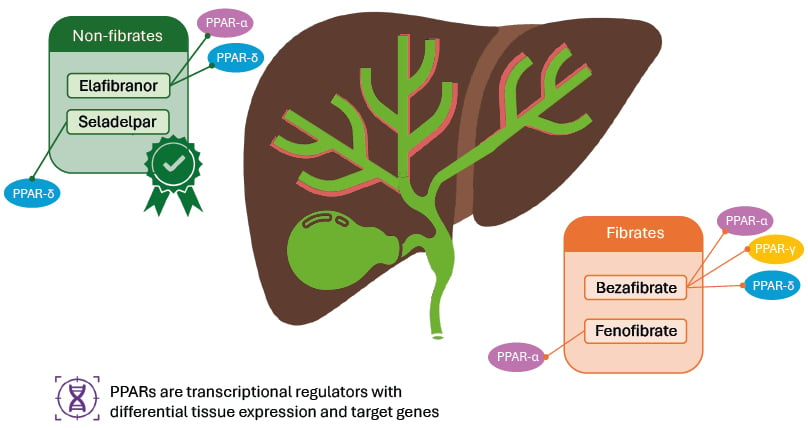
Figure 1: Peroxisome proliferator-activated receptor agonists in primary biliary cholangitis.
Adapted from Colapietro F et al.27 2023.
The green box indicates PPAR agonist approved for use in PBC;8,9 the orange box indicates PPARs commonly used off-label in PBC.
PPAR: peroxisome proliferator-activated receptor; PBC: primary biliary cholangitis.
Bezafibrate is a pan-PPAR agonist that has been shown to be effective (off-label) in PBC.1 An approved therapy for dyslipidaemia, studies evaluating bezafibrate in PBC have demonstrated improvements in liver biochemistry, liver stiffness, and pruritus in patients with incomplete responses to UDCA.28 28 However, bezafibrate is not approved for the treatment of PBC, and therefore the latest European Association for the Study of the Liver (EASL) guidelines (2017) do not offer recommendations for its use in this condition.10 Similarly, fenofibrate, predominantly a PPARα agonist, is used (off-label) in PBC in some countries (mainly in the USA, where bezafibrate is not available), and retrospective observational studies have demonstrated improved liver biochemistry, bile acid levels, and pruritus in combination therapy with UDCA.29,30
Two new PPAR agonists were approved for the treatment of PBC in 2024 following positive results from Phase III clinical trials.8,9 In the EU, elafibranor and seladelpar are both indicated for the treatment of PBC in combination with UDCA in adults who have an inadequate response to UDCA alone, or as monotherapy in those unable to tolerate UDCA.8,9
Elafibranor is a dual agonist of PPARα and PPARδ, isoforms that are thought to be key regulators of bile acid homeostasis, inflammation, and fibrosis.8 Activation of these receptors decreases bile toxicity and improves cholestasis.8 Seladelpar is a selective PPARδ agonist; activation of this receptor reduces bile acid synthesis, reducing hepatic bile acid exposure and reduced circulating bile acid levels.9
The approval of elafibranor and seladelpar were based on Phase III trials in patients with PBC who had an inadequate response to, or a history of unacceptable side effects with, UDCA.18,31 The Phase III ELATIVE trial evaluated elafibranor 80 mg once per day versus placebo in 161 patients with PBC,18 and the Phase III RESPONSE trial evaluated seladelpar 10 mg once per day versus placebo in 193 patients with PBC.31 Patients with advanced cirrhosis were excluded.18,31 The vast majority of patients in each trial continued to receive UDCA as background therapy.18,31 The primary endpoint for both trials was a biochemical response, defined as ALP <1.67 times the ULN and ≥15% decrease from baseline, and a normal total bilirubin level.18,31
Primary Efficacy Endpoint
Results for the primary endpoint of these trials showed that both PPARs were effective at reducing liver biochemistry.18,31 At Month 12 in ELATIVE, 51% of patients treated with elafibranor had achieved a biochemical response versus 4% of patients receiving placebo (difference: 47 percentage points; 95% CI: 32–57; p<0.001).18 At Month 12 in RESPONSE, 61.7% of patients treated with seladelpar had achieved a biochemical response versus 20% of patients receiving placebo (difference: 42 percentage points; 95% CI: 27.7–53.4; p<0.001).31 Berg pointed out that the response was quite rapid in both trials, with the most pronounced decline in ALP occurring within the first 4 weeks.18,31
All the experts interviewed agreed that it was difficult to compare the efficacy of elafibranor and seladelpar at this stage, because of differences in study design and population between the clinical trials and limited real-world data to draw from. Similarly, Berg stressed that while both PPAR agonists appeared to have positive effects on pruritus,18,31 it is difficult to say whether one is superior to the other. In addition to confirming the efficacy of the new medicines, Trivedi explained that real-world data will help to determine how well they work alongside other licenced therapies.
Numerically more patients achieved a biochemical response with seladelpar in RESPONSE than elafibranor in ELATIVE (62% and 51%, respectively).18,31 However, more patients in the placebo arm of RESPONSE also achieved a biochemical response than in ELATIVE (20% and 4%, respectively), indicating a fundamental difference between the trial populations.18,31
Secondary Efficacy Endpoints
Another difference between the active arms of the two trials was the proportion of patients with normalised ALP, which Trivedi emphasised is increasingly a goal of therapy in PBC.22 In ELATIVE, 15% of patients treated with elafibranor achieved this target, whereas 25% achieved it with seladelpar in RESPONSE.18,31 No patients achieved this target with placebo in either trial.18,31
Both trials included an evaluation of pruritus as a secondary endpoint, although Trivedi noted that only the RESPONSE trial was powered to show significance for this endpoint.18,31 RESPONSE showed that after 12 months, seladelpar resulted in a greater reduction in the pruritus numerical rating scale (NRS) score from baseline versus placebo (least-squares mean [LSM] change: -3.2 versus -1.7; difference: -1.5; 95% CI: -2.5–-0.5; p=0.005).31 Among patients who had moderate-to-severe pruritus at baseline in ELATIVE, the worst itch NRS did not differ significantly between those receiving elafibranor versus placebo (LSM change: -1.93 versus -1.15; difference: -0.78; 95% CI: -1.99–0.42; p=0.20).18 However, further analyses of ELATIVE showed that QoL measures such as the PBC-40 quality of life questionnaire (LSM difference: -2.3; 95% CI: -4.0–-0.7) and the 5-D itch scale (LSM difference: -3.0; 95% CI: -5.5–-0.5) indicated a possible reduction in pruritus with elafibranor versus placebo, respectively.18
Additional Efficacy Analyses
Berg and Trauner pointed out data from an interim analysis of the ongoing ELATIVE open-label extension (OLE) that indicated long-term treatment with elafibranor resulted in clinically meaningful improvements in fatigue and sleep in patients with PBC. Among patients who received elafibranor in both the parent trial and the OLE (n=48), from baseline to Week 104, 56% had a ≥3-point improvement in the Patient-Reported Outcome Measurement Information System (PROMIS) Fatigue Short Form 7a score, and 69% had a ≥2-point improvement in excessive daytime sleepiness score.32 A Phase II study has shown that seladelpar may have similar benefits in terms of fatigue, with 64% of patients treated with seladelpar 10 mg experiencing improvement in PBC-40 fatigue scores;33 however, fatigue data from RESPONSE has yet to be published at the time of writing.
Several other efficacy analyses of ELATIVE and RESPONSE were presented at the American Association for the Study of Liver Diseases (AASLD) Liver Meeting 2024.34-38 Data for elafibranor indicated that treatment was associated with improvements in predicted transplant-free survival based on GLOBE and UK-PBC scores,34 and that improvements in cholestasis and pruritus were sustained up to Week 156 in the ELATIVE OLE study.35 Data for seladelpar indicated that treatment reduced biomarkers of cholestasis and liver injury in patients with and without cirrhosis,36 led to near resolution of itch in some patients and mitigated new onset of itch,37 and reduced ALP regardless of baseline ALP level across subgroups that included patients aged <50 years at diagnosis and those with Hispanic/Latino ethnicity.38
Berg pointed out that one of the efficacy analyses of RESPONSE showed that 26.7% of patients receiving placebo developed new pruritus during the trial, compared with none of the patients receiving seladelpar, indicating that the longer PBC is left untreated, the higher the risk of developing symptoms.37
Safety
Neither the addition of elafibranor nor seladelpar to UDCA increased the frequency of adverse events (AE) overall in the ELATIVE and RESPONSE trials.18,31
In ELATIVE, AEs that were reported more frequently in the elafibranor group versus the placebo group included abdominal pain (11% versus 6%, respectively), diarrhoea (11% versus 9%), nausea (11% versus 6%), and vomiting (11% versus 2%). The majority of AEs were of mild or moderate intensity. Elevated serum creatinine levels and muscle injury were also more common in the elafibranor group than the placebo group (≥25% above baseline in 10.2% versus 7.5% of patients, respectively), resulting in discontinuation of treatment in four patients (3.7%) in the elafibranor group. Elevations were associated with myalgia in two patients, and an additional patient with advanced cirrhosis receiving concomitant atorvastatin (40 mg once daily) experienced a serious case of rhabdomyolysis. Fatal AEs occurred in two patients (1.9%) receiving elafibranor; neither was considered by investigators or an independent clinical events committee to be related to treatment.18 In an additional safety analysis of ELATIVE, no clinically meaningful changes in renal function were observed with elafibranor versus placebo.39
In RESPONSE, AEs that were reported more frequently in the seladelpar group versus the placebo group included COVID-19 (18% versus 15.4%, respectively), headache (7.8% versus 3.1%), abdominal pain (7% versus 1.5%), nausea (6.2% versus 4.6%), and abdominal distention (6.2% versus 3.1%); these AEs were mild or moderate in severity and did not result in treatment discontinuation. The safety profile was similar in patients who did and did not have cirrhosis at baseline.31
Long-term pooled safety data for seladelpar were presented at AASLD 2024,40 showing that this PPAR agonist remained generally well tolerated. No new safety signals were identified in an interim analysis of the ELATIVE OLE up to 3 years,35 and treatment with seladelpar 10 mg up to 5 years showed a similar safety profile to placebo, in a pooled analysis of six studies.40 Analysis of pooled data also showed that there were no safety concerns in patients co-administered statins and seladelpar, and that seladelpar was associated with reductions in total cholesterol and low-density lipoprotein cholesterol over 12 months of treatment.41
Trauner emphasised that increases in serum creatinine were expected with elafibranor, since these are known to occur with other PPARα agonists.42 He also pointed out that elafibranor may show interactions with the metabolism of oral contraceptives and, based on pre-clinical studies, is contraindicated in pregnancy or in women who may become pregnant and do not use non-hormonal contraceptive methods, whereas seladelpar has not been shown to be teratogenic, although its use in pregnancy is also not recommended.8,9 Trauner explained that “…this [impacts] just a minority of patients, …[but] the less you have to worry about, the better.”
The off-label use of bezafibrate with UDCA is associated with increases in creatinine and myalgia; in the BEZURSO trial, serum creatinine increased from baseline by 5% in the bezafibrate group and decreased by 3% in the placebo group, and myalgia occurred in 20% and 10% of patients in each group, respectively.43 Trauner stressed that overall, data from ELATIVE and RESPONSE show that both elafibranor and seladelpar are effective treatments that may be better tolerated than bezafibrate.
THE FUTURE OF PRIMARY BILIARY CHOLANGITIS TREATMENT
All three of the experts interviewed felt that the approval of elafibranor and seladelpar would change their clinical practice. Berg explained that although the use of fibrates, such as bezafibrate, were a “big step forward” in PBC, in his personal experience, approximately 10–20% of patients discontinue treatment due to side effects such as muscle pain or general malaise (Berg, Personal communication).44 He emphasised that clinical trial data for elafibranor and seladelpar suggest a better safety profile compared with fibrates. Trivedi added that rare but serious cases of drug-induced liver injury have also been associated with fibrates,45 and that real-world data for elafibranor and seladelpar will be needed to validate clinical trial observations that these more selective PPARs are not associated with liver injury.
Trivedi stressed that for patients with an inadequate response to UDCA alone and who also experience pruritus, he would previously have prescribed bezafibrate (off-label) but will now prescribe elafibranor or seladelpar. Trauner explained that when selecting one of the two newly licenced PPARs for his patients, he will consider the safety profiles of the PPARs against patient-specific factors such as the need for statins, the risk of kidney disease, and the presence of pruritus or fatigue. Berg emphasised the advantages of being able to offer patients therapies that, in addition to disease control, may improve their quality of life.
Considerations of Primary Biliary Cholangitis Stage
The ELATIVE and RESPONSE trials included patients with early cirrhosis; however, those with more advanced cirrhosis were excluded.18,31 In ELATIVE, patients were excluded if they had total bilirubin >2 times the ULN or clinically significant hepatic decompensation.18 In RESPONSE, patients were excluded if they had advanced PBC (albumin below the lower limit of normal and total bilirubin above the ULN), hepatic decompensation, or any other chronic liver disease.31
Berg stressed that he would be cautious about treating patients with more advanced PBC, because of the lack of data for this population. He also explained that, since bile duct loss in PBC is permanent, more real-world data are needed to determine whether either of the new PPAR agonists could change the course of the disease at this later stage. Trivedi agreed that caution was sensible in patients with advanced PBC. “We are still unclear and a little bit pensive about offering [PPAR agonist] treatment [to these patients],” he said. “I wouldn’t say it is a practice I would advocate outside of a transplant centre.”25
Changes to Guidelines
All of the experts felt that the approval of elafibranor and seladelpar are likely to trigger an update to PBC practice guidelines. Aside from the need to include these options in the treatment paradigm, Trauner felt that guidelines need to bring in a more personalised approach to therapy. For example, whether the treatment target of normalisation of ALP is appropriate for all patients, or whether is more suited to younger patients with advanced fibrosis and less to patients >60 years of age without significant fibrosis. He also felt that, now that there are more options for patients with PBC, it may be worth including guidance on selection criteria, such as comorbidity and symptoms. Trivedi agreed, adding that normalisation of ALP, particularly for patients with advanced fibrosis or of a young presenting age, has been associated with increased complication-free survival rate compared to lowering biochemistry to a biochemical response threshold.22,23
Future Treatment Prospects
Berg, Trauner, and Trivedi described some of the treatments being evaluated for PBC, and shared their perspectives on the impact they might have on the future management of the disease.
Ileal Bile Acid Transporter Inhibitors for Pruritus
Bile acids are one of the pruritogenic substances thought to be responsible for itch symptoms in cholestatic disease,21 and ileal bile acid transporter (IBAT) inhibitors, which interrupt the uptake of bile acid in the small intestine, are being evaluated for PBC-associated pruritus.46 Updated data for the investigational IBATs linerixibat and volixibat were presented at AASLD 2024.46-48
Post-hoc analysis of a Phase IIb trial in patients with PBC and pruritus (GLIMMER) suggested that linerixibat was associated with a dose-dependent reduction in itch,49 and a Phase III trial (GLISTEN) completed at the end of 2024.50 Though results have yet to be published, an early press release reported that GLISTEN met its primary endpoint, with a statistically significant improvement in itch over 24 weeks versus placebo.51
Similarly, volixibat is currently undergoing evaluation in a Phase IIb trial in PBC (VANTAGE).47 Interim data suggest that volixibat may lead to early and significant reductions in PBC-associated pruritus and fatigue.47 Trivedi emphasised that IBATs are likely to become important adjunctive therapies that add to the anti-pruritic armoury in PBC.
Golexanolone for Cognitive Symptoms
Neuro-steroids, such as allopreganolone, have been shown to be elevated in patients with PBC who experience cognitive symptoms.52 Golexanolone, a novel oral GABAA receptor-modulating steroid antagonist, has shown promise in ameliorating cognitive and sleep disorders induced by allopreganalone.53 Golexanolone is currently being evaluated in an ongoing Phase I/II trial in patients with PBC who experience significant fatigue and cognitive symptoms.54 Initial data from this trial are reported to be promising,53 and Trivedi stressed that this is one of the first investigative agents to target cognitive impairment in PBC.
Setanaxib for Fatigue
Setanaxib is a selective inhibitor of NADPH oxidase (NOX) 1 and 4 isoforms, with anti-fibrotic and anti-inflammatory effects that prevent progression to liver fibrosis in pre-clinical studies.55 Trauner considered this an interesting, novel approach to PBC therapy. A Phase II trial of setanaxib plus UDCA in patients with PBC was designed with a primary efficacy endpoint of percentage change in GGT from baseline to Week 24, since GGT is a marker of inflammation and oxidative stress.55 While the trial failed to demonstrate a statistically significant difference between setanaxib and placebo for the primary endpoint, secondary endpoints were more promising, supporting further investigation. In particular, clinically significant improvements in fatigue were noted in patients receiving setanaxib versus placebo,55 and post-hoc analyses confirmed that these improvements correlated with emotional, social, and cognitive improvements.56
CONCLUSION
Further research will hopefully be able to answer some of the questions raised by Berg, Trauner, and Trivedi. For example, in vitro studies and a deeper understanding of biomarkers may help us to better understand the pathogenesis of PBC, and the drivers of disease progression and biliary injury. Treatment of patients with selective PPAR agonists may help us to learn how different PPARs influence chronic liver disease, and therefore which medications are likely to be most effective and well-tolerated in individual patients. It will also be important to evaluate how the new PPAR agonists interact with concomitant therapies such as OCA (off-label in the EU), to determine whether some patients might benefit from combining these drugs versus switching between them.
The interviewed experts agreed that the approval of elafibranor and seladelpar for PBC were a positive step forward for the management PBC. “These agents are producing a durable biochemical response without an adverse safety signal”, emphasised Trivedi, “…these two treatments are going to revolutionise PBC care going forward, and it’s absolutely fantastic that patients have more options now.”
| Elafibranor and Seladelpar: Adverse events should be reported. Healthcare professionals in the UK, can report via the Yellow Card Scheme found at yellowcard.mhra.gov.uk or search for MHRA Yellow Card in the Google Play or Apple App Store. Healthcare professionals outside of the UK should refer to local prescribing information and report any suspected adverse reactions via the national reporting system listed in Appendix V. |







