Abstract
Secondary bacterial peritonitis is a life-threatening disease requiring early distinction from spontaneous bacterial peritonitis due to shared features such as fever, leukocytosis, and abdominal pain. Despite appropriate antibiotic therapy, persistent fever, leukocytosis, and culture-positive fluid should always prompt further investigation for a secondary source of infection.
The authors present a rare case of a patient with cirrhosis who had an untreated perinephric abscess and developed antibiotic-resistant peritonitis, raising concerns of secondary bacterial peritonitis arising from the abscess. The patient presented to the emergency department with malaise, nausea, and vomiting in the context of a recent admission 2 months prior for a urinary tract infection caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae, which is sensitive to ertapenem. This was complicated by the development of multiple renal abscesses. At the time, the patient received a percutaneous drain in his largest renal abscess and was discharged with an outpatient ertapenem infusion scheduled 1 month later, but he was unable to follow up. Despite adequate antibiotic therapy, peritoneal drainage demonstrated persistent culture-positive fluid and elevated neutrophils, raising suspicion of a secondary source of peritonitis that ultimately required surgical intervention. This case emphasises the importance of early suspicion of a secondary source of peritonitis in patients who demonstrate persistent fever, leukocytosis, and culture-positive peritoneal fluid despite adequate antibiotic therapy, and a difference in management for spontaneous bacterial peritonitis versus secondary peritonitis.
Key Points
1. Secondary peritonitis is a surgical emergency with a mortality rate approaching 20%.2. This case report outlines a rare case of a patient with cirrhosis who had an untreated perinephric abscess and developed antibiotic-resistant peritonitis, raising concerns of secondary bacterial peritonitis arising from the perinephric abscess.
3. Persistent fever, leukocytosis, and culture-positive fluid despite appropriate antibiotic therapy should initiate further investigations for a secondary source of infection, given the high morbidity caused by a lack of prompt treatment for this condition. Surgery is necessary for patients with secondary bacterial peritonitis.
INTRODUCTION
Bacterial peritonitis can either arise spontaneously or occur secondary to abscesses or perforations in the abdominal cavity. Both aetiologies present similarly with fever, leukocytosis, and abdominal pain, but a full response to antibiotics is expected in spontaneous peritonitis. Persistent fever, leukocytosis, and culture-positive fluid despite antibiotic therapy should prompt further investigation for a secondary source of infection, which requires surgical treatment.
Spontaneous bacterial peritonitis is characterised by an infection of ascitic fluid that does not have an evident intra-abdominal source amenable to surgery.1 An absolute polymorphonuclear leukocyte count of at least 250 cells/cmm defines the presence of infection.2 The most common causative pathogens, in terms of isolated species from ascitic fluid cultures, are Escherichia coli (33%), Streptococcus (15%), Klebsiella (13%), and Enterococcus (9%).2 The most common predisposing condition to spontaneous bacterial peritonitis is cirrhosis of the liver. In cirrhosis, spontaneous bacterial peritonitis is thought to occur due to decreased liver function and increased pressure in the portal system. As a result of these factors, ascitic fluid builds up in the abdominal cavity, and the passage of intestinal bacteria into intestinal lymph vessels, systemic circulation, and ascitic fluid can occur, leading to infection.3
While secondary bacterial peritonitis has several similarities to spontaneous bacterial peritonitis in terms of clinical presentation (fever, leukocytosis, abdominal pain), the aetiology is different. As previously mentioned, spontaneous bacterial peritonitis most commonly presents in patients with cirrhosis and subsequent ascites. The aetiologies of secondary bacterial peritonitis, however, usually stem from a perforation in the gastrointestinal system or from abdominal abscesses.4,5 Furthermore, early recognition of secondary bacterial peritonitis is imperative, as the treatment significantly differs from spontaneous bacterial peritonitis.Surgery is the primary treatment of secondary bacterial peritonitis, given the most common aetiologies, whereas in spontaneous bacterial peritonitis, surgery is not considered.6,7 Another important consideration is that, in patients with cirrhosis, there is an increased risk of mortality with laparoscopic procedures.8 An unnecessary laparotomy in a patient with spontaneous bacterial peritonitis has an approximately 80% mortality rate.9 A patient with secondary bacterial peritonitis has a near 100% mortality rate if they are treated with antibiotics alone and surgery is not performed to address the source of infection.4
Here, the authors present a patient with a past medical history of cirrhosis and an incompletely treated renal abscess, who later developed peritonitis that failed to improve despite adequate antibiotic therapy, leading to suspicion of bacterial peritonitis secondary to the renal abscess.
CASE PRESENTATION
A 40-year-old male presented to the emergency department with malaise, nausea, and vomiting. Past medical history revealed that he was admitted 2 months prior for a urinary tract infection caused by extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae, which is sensitive to ertapenem. This was complicated by the development of multiple renal abscesses and alcoholic cirrhosis with minimal ascites on previous admission. The patient received a percutaneous drain in his largest renal abscess and was discharged with an outpatient ertapenem infusion scheduled 1 month later, with plans for repeat imaging to monitor the resolution of his abscesses. However, the patient had not followed up in the outpatient setting or received his ertapenem infusion. He was admitted for concerns of sepsis and was found to have a leukocytosis of 21,000 cells/cmm (reference: 4,500–11,000 cells/cmm), an elevated lactic acid of 4.5 mmol/L (reference: 0.50–2.2 mmol/L), and tachycardia of 140 beats per minute. Urine culture demonstrated the growth of ESBL-producing Klebsiella pneumoniae. Blood cultures did not show the growth of any organisms. The abdominal and pelvic CT demonstrated lobulated areas of fluid density along the right kidney, with the largest being 4.18 cm; a moderate amount of intra-abdominal fluid with a possible loculated component; and peritoneal hyperenhancement that was concerning for possible peritonitis (Figure 1). Diagnostic paracentesis demonstrated a white blood cell count of 2,875 cells/cmm (reference: 0–10 cells/cmm) that was 95% neutrophils, with the growth of ESBL-producing Klebsiella pneumoniae sensitive to ertapenem. The patient received a repeat placement of a renal percutaneous drain that demonstrated recurrent growth of ESBL-producing Klebsiella pneumoniae sensitive to ertapenem. There was persistent leukocytosis despite ertapenem therapy and drainage of the abscess, pointing towards a diagnosis of secondary peritonitis rather than spontaneous bacterial peritonitis. Given concerns for possible intraperitoneal loculations, the patient underwent a repeat paracentesis along with the placement of a peritoneal drain. One litre of frank pus was removed, which demonstrated persistent growth of ESBL-producing Klebsiella pneumoniae sensitive to ertapenem, with a white blood cell count of 408,000 cells/cmm (reference: 0–10 cells/cmm) that was 100% neutrophils. General surgery was consulted to discuss surgical abdominal washout, but the patient was deemed a poor surgical candidate due to him having Child-Pugh Class C cirrhosis, which corresponded to an estimated perioperative mortality of 82%. A repeat abdominal and pelvic CT demonstrated persistent loculated fluid collections with repeat peritoneal drain placement, demonstrating purulent fluid and growth of ESBL-producing Klebsiella pneumoniae sensitive to ertapenem. The patient initially demonstrated transient improvement following drainage of the loculated collection, but his condition subsequently worsened. Given the patient’s high surgical risk, repeat percutaneous drainage attempts were made, but with inadequate drainage and continued deterioration, he developed septic shock requiring vasopressor support. He then underwent a laparotomy and an abdominal washout procedure with clearance of multiple purulent pockets. The postoperative course was complicated by bleeding, requiring a blood transfusion and tranexamic acid administration. Following the blood transfusion, the patient demonstrated marked clinical improvement and was weaned off vasopressors. The patient was deemed stable for transfer to a long-term acute care facility for continued ertapenem infusion along with outpatient follow-up with general surgery and infectious disease.
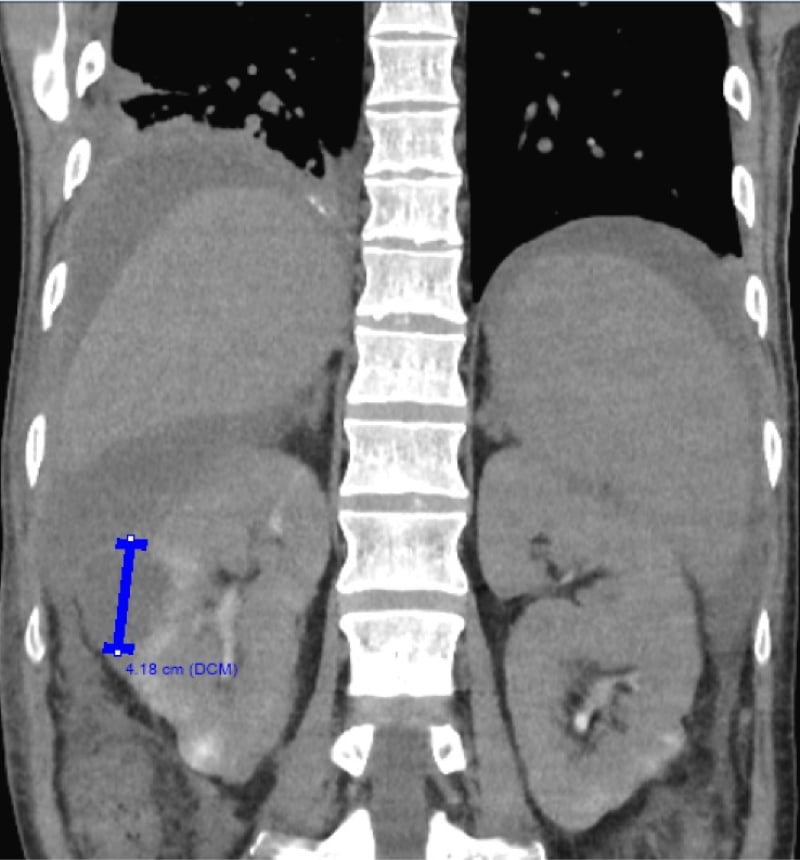
Figure 1: Abdominal and pelvic CT.
An abdominal and pelvic CT demonstrating multiple lobulated areas of fluid density located along the right kidney. The largest discrete lesion measures 4.18 cm and is delineated with a blue line.
DISCUSSION
Bacterial peritonitis poses clinical challenges that must be addressed to decrease the chance of further complications. Two aetiologies include spontaneous bacterial peritonitis and secondary bacterial peritonitis. Both share some similarities, but it is crucial to highlight how they differ in aetiology, patient presentation, and treatment to prevent a poor prognosis. Understanding these differences can guide clinical management and prevent misdiagnoses in the future.
A low index of suspicion should be held for spontaneous bacterial peritonitis in patients with cirrhosis and ascites. The most common presenting symptoms are fever, chills, abdominal pain, and confusion, although some patients may be entirely asymptomatic.10 At times, the presence of ascites can make abdominal pain less obvious, and some patients might even present without abdominal pain.11 Early recognition is essential to increase the likelihood of a positive prognosis because, if not treated in time, spontaneous bacterial peritonitis can lead to septic shock with resultant organ failure. Kumar A et al.4 highlight the decreased survival rate in patients with sepsis for each hour without antibiotic treatment. Within the first hour of documented hypotension, if proper antibiotic treatment was administered, there was a 79.9% survival rate.4 Once a diagnosis of spontaneous bacterial peritonitis has been made via paracentesis, patients should be initiated on empiric antibiotic treatment, such as with a third-generation cephalosporin, until their cultures come back with a specific pathogen, at which time antibiotic treatment can be tailored accordingly.11
Discerning between the two conditions accurately can mean the difference between life and death for a patient. Thus, it is prudent to be aware of the signs of secondary bacterial peritonitis so that appropriate measures can be taken. In the authors’ case, sepsis was a major concern during the patient’s second admission, and a follow-up abdominal and pelvic CT revealed intra-abdominal fluid with findings that were concerning for peritonitis. The patient received ertapenem therapy as well as placement of a renal percutaneous drain due to lobulated densities along the right kidney. Even with the appropriate antibiotic treatment, the patient continued to be febrile and demonstrated persistent leukocytosis, something that would be unexpected in spontaneous bacterial peritonitis. His condition did not improve until surgery was performed, pointing towards a diagnosis of secondary peritonitis. While reviewing the literature, another case report about a patient with secondary bacterial peritonitis noted that consistent temperature spikes and increased leukocyte counts in the setting of appropriate antibiotic therapy should raise concern for secondary peritonitis,11 as was seen in this patient. In this case, Runyon11 notes that secondary peritonitis via perforations tends to be polymicrobial due to gut flora leakage into the abdominal cavity. The patient was found to have a recurrent monomicrobial infection with ESBL-producing Klebsiella pneumoniae, which would be a supportive clinical manifestation of a localised abscess instead of an intestinal perforation.
LITERATURE REVIEW
Secondary peritonitis is a rare but serious complication in patients with cirrhosis, accounting for 4.5% of all cases of peritonitis in this cohort of patients and having a mortality rate between 50–80%.12,13 Given its similar presentation and starkly different treatment to the more commonly occurring spontaneous bacterial peritonitis, early recognition is necessary to reduce mortality. While spontaneous bacterial peritonitis is treated with antibiotic therapy, secondary bacterial peritonitis requires surgical removal of the infectious source in addition to antibiotic therapy.6,7 Secondary bacterial peritonitis usually stems from a perforation in the gastrointestinal system or from an abdominal abscess, with one study finding that the most common sources of infection were the colon or rectum followed by the stomach or duodenum.4-6 This case reports a source of infection that, to the authors’ knowledge, has not been previously reported in other literature: a perinephric abscess, secondary to an untreated ESBL urinary tract infection from a prior hospital admission. This case challenges current knowledge behind common sources of secondary peritonitis, and highlights the poorly understood link between retroperitoneal abscesses and secondary peritonitis.
CONCLUSION
The aetiology of secondary peritonitis is traditionally categorised into either gastrointestinal tract perforation or an abdominal abscess without evidence of perforation. This case revealed a previously unreported origin: a retroperitoneal abscess, as the source of the patient’s peritonitis was a right perinephric abscess. Despite ertapenem therapy, peritoneal fluid drainage demonstrated persistent growth of ESBL-producing Klebsiella pneumoniae. This would be a phenomenon rarely seen in the setting of spontaneous bacterial peritonitis, as most cases show culture-negative therapy ascitic fluid upon the initiation of appropriate antibiotic treatment. Persistent fever, leukocytosis, and culture-positive fluid, despite appropriate antibiotic therapy, should initiate further investigation for a secondary source of infection, particularly given the high morbidity that can occur from a lack of prompt treatment for this condition. Surgery is necessary for patients with secondary bacterial peritonitis.
INFORMED CONSENT
Written informed consent was obtained from the patient’s next of kin for this case report, as at the time of drafting the manuscript, the patient had unfortunately passed away.







