BACKGROUND AND AIMS
Critical limb ischaemia (CLI) remains a major challenge in patients with peripheral artery disease (PAD),1 particularly when chronic total occlusions (CTO) complicate endovascular revascularisation. Subintimal recanalisation of long CTOs could be challenging, with a risk of failure as high as 25%, necessitating more complicated retrograde recanalisation via distal puncture or surgical conversion.2 Recently, a new dedicated dual guidewire angioplasty balloon (the Presto balloon, APANI Medical Inc, California, USA) was introduced to perform antegrade fenestration re-entry (AFR), with the aim of improving clinical outcomes and reducing procedural times and X-ray exposure.3
The purpose of this study was to evaluate the 36-month safety and efficacy of AFR using the Presto balloon for CTO crossing in patients with CLI, compared with conventional subintimal recanalisation, in the PRAESTO Trial.4
METHODS
This retrospective, score-matched analysis included 392 patients with PAD and CTO. A propensity score analysis, based on cardiovascular risk factors and lesion characteristics, was performed to create two homogenous groups: 40 patients underwent AFR with the Presto device (study group), while 80 matched controls were treated with standard subintimal recanalisation techniques (control group). The assessed outcomes included procedural success, subintimal re-entry rates, need for distal puncture, procedural and fluoroscopy times, radiation exposure (measured by dose area product), and long-term clinical endpoints (primary patency, freedom from amputation, re-intervention, and death).
RESULTS
AFR using the Presto balloon achieved a significantly higher subintimal re-entry success rate (100% versus 78.7%; p=0.001), and eliminated the need for retrograde tibial access (0% versus 21%; p=0.001). Surgical bypass conversion was required in five control patients. The Presto balloon study group demonstrated significantly shorter procedural times (41.6±11.4 minutes versus 139.8±61 minutes; p<0.0001) and fluoroscopy times (16.9±3.5 minutes versus 32.8±14 minutes; p<0.0001), with drastically lower dose area product (942.2±290 µGym² versus 1935.7±830.1 µGym²; p<0.0001). At 36 months, the Presto study group showed improved freedom from amputation (p=0.04) and re-intervention (p=0.03), with no significant difference in mortality between groups (Figure 1).
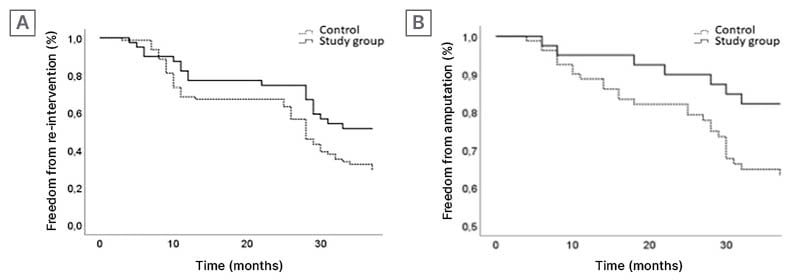
Figure 1: Kaplan–Meier curves representing the freedom from re-intervention and amputation over time.
A) Kaplan–Meier curve representing the 36-month follow-up freedom from re-intervention. B) Kaplan–Meier curve representing the 36-month follow-up freedom from amputation.
CONCLUSION
AFR with the Presto dual guidewire angioplasty balloon is a safe and effective method for CTO crossing in patients with PAD and CLI. This technique significantly reduces procedural and fluoroscopy times, radiation exposure, and the need for distal puncture, while improving long-term outcomes related to amputation and re-intervention. These benefits are achieved without increasing procedural risks, suggesting a valuable advancement in endovascular CLI treatment.