BACKGROUND AND AIMS
Natural killer (NK) cells are increasingly studied in HIV cure research as certain subsets have been linked to natural HIV control, enhanced antibody-dependent cellular cytotoxicity (ADCC), and reduced viral reservoirs.1-6 Elite controllers (EC) often display higher envelope (Env)-specific ADCC activity,7 and the only moderately successful HIV vaccine trial to date, RV144, identified ADCC as a correlate of reduced acquisition risk.8
METHODS
Based on these findings, the authors performed in-depth phenotyping and functional testing of NK cells from the second Berlin individual (B2), who displayed sustained HIV remission after allogeneic stem cell transplantation with heterozygous CCR5Δ32 donor cells. Peripheral blood mononuclear cells were genotyped for killer-Ig-like receptor genes, and NK cells were analysed by multiparameter flow cytometry, as well as standardised ADCC assays, against rituximab-coated target cells. HIV-1-specific ADCC capacity was tested in longitudinal plasma samples from B2, employing a degranulation assay against immobilised Env protein, in comparison with plasma from ECs and broadly neutralising antibodies (Figure 1).
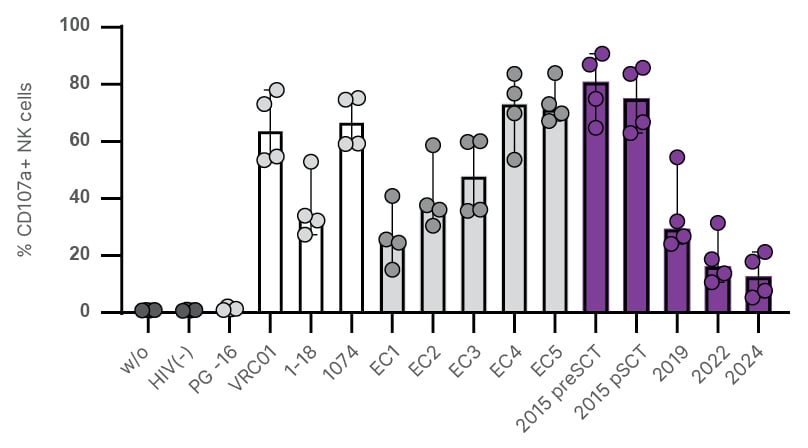
Figure 1: HIV-specific antibody-dependent cellular cytotoxicity activity of the second Berlin individual’s plasma.
Summary of cumulative ADCC responses. NK cells (N=4 biological replicates) were incubated with Env-coated wells treated with PBS, plasma from a donor who is HIV-negative (1:1000 dilution; black), bnAbs (PG-16, 1–18, VRC01, and 10–74; 10 µg/mL; white), plasma from five ECs (1:1000; dark grey), or longitudinal B2 plasma samples (1:1000 dilution; purple). The bar shows the median with the IQR.
ADCC: antibody-dependent cellular cytotoxicity; B2: second Berlin individual; bnAbs: broadly neutralising antibodies; CD: cluster of differentiation; EC: elite controllers; Env: envelope; IQR: interquartile range; NK: natural killer; PBS: phosphate-buffered saline; SCT: stem cell transplant; w/o: without.
RESULTS
B2 displayed an NK cell phenotype with a markedly higher frequency of natural killer group 2 member A (NKG2A)⁺ cluster of differentiation (CD)57⁺ NK cells compared to controls who were HIV-negative and cytomegalovirus-positive, and previously published data on allogeneic stem cell transplantation recipients.9 This subset did not undergo downmodulation of promyelocytic leukaemia zinc finger and Fc ɛ receptor γ chain characteristics of adaptive-like NK cells, but nonetheless contributed substantially to ADCC-activity upon encountering rituximab-coated targets. Around transplantation, B2’s plasma mediated high HIV-specific ADCC activity, surpassing EC plasma (N=5) and monoclonal broadly neutralising antibodies. ADCC activity mediated by B2’s plasma declined substantially in the years following transplantation (Figure 1).
CONCLUSION
Taken together, the authors observed an NK cell phenotype in B2 characterised by the expansion of mature NKG2A+ NK cells, alongside potent HIV-specific plasma ADCC activity. These data, together with findings from the first case of HIV-1 remission after transplantation with wild-type CCR5 donor cells (the Geneva Case),10 suggest a potential contribution of NK cell-mediated mechanisms to modulate the HIV reservoir, independent of CCR5Δ32 homozygosity.







