Interview Summary
The HIV treatment landscape is evolving, ushering in the era of long-acting (LA) antiretroviral therapy (ART) to support the challenges that still persist in HIV management. LA therapies can support an HIV-free generation, offering benefits like improved adherence, better quality of life (QoL), reduced stigma impact, and redefining treatment success. Through evidence-based solutions and clinical expertise, three global infectious disease experts shared insights from case studies presented at the ViiV-sponsored symposium during the European AIDS Clinical Society (EACS) 2025 Conference. These case studies highlight key issues in HIV care, including adherence challenges, comorbidities, weight gain, and ageing. The leading HIV experts who provided their input and perspectives were Karine Lacombe, Professor in the Faculty of Health Sciences at Sorbonne University and Head of the Department of Infectious and Tropical Diseases at Saint-Antoine Hospital in Paris, France; Monica Gandhi, Professor of Medicine and Medical Director of the HIV Clinic in the Division of HIV, Infectious Diseases and Global Medicine at the University of California San Francisco (UCSF), USA; and Chloe Orkin, Professor of HIV/AIDS Medicine at Queen Mary University of London, UK.Drawing on the successes of LA in psychiatry, diabetes, and oncology, HIV care is now following a similar path towards optimised patient outcomes through the use of these therapies. The objective of this article is to highlight the expanding role of LA injectables in HIV management and discuss, with expert insights, how the use of LAs can help address some of the main challenges facing clinicians and patients living with HIV today.
CASE STUDY 1: CARE AND COMORBIDITIES – MANAGING AGEING PEOPLE LIVING WITH HIV
Maria de Legarde, Hospital Universitario 12 de Octubre, Madrid, Spain presented the case of Pepe, a 58-year-old man who has sex with men (MSM), lives alone in Madrid, Spain, and works as a teacher. He is at high cardiovascular (CV) risk and has multiple comorbidities, including osteoporosis and major chronic depression. Pepe has had significant mental health issues over a substantial period of time and engages in chemsex, which has resulted in several sexually transmitted infections (STI). Baseline clinical parameters were as follows:
- Labs and assessments: decreased high-density lipoprotein (HDL): 38 mg/dL; increased low-density lipoprotein (LDL): 158 mg/dL; increased total cholesterol: 5.0 mmol/L; increased BMI: 29.9 kg/m2; decreased estimated glomerular filtration rate: 85 mL/min/1.73 m².
- Comorbidities: osteoporosis (2016); chronic gastritis, Helicobacter pylori (2005) treated; depression with impulses (2018); hepatitis C (genotypes 1a + 3, successfully treated and RNA negative).
- Drug history: fenofibrate plus ezetimibe; calcium plus vitamin D; lansoprazole; venlafaxine; quetiapine.
Pepe was diagnosed with HIV in 2004 with a viral load of 449,310 copies/mL and a CD4+ T cell count of 252 cells/mm3. His treatment history has featured multiple changes in the ART regimen:
- May 2004: Initiated zidovudine/lamivudine (3TC) plus efavirenz in a clinical trial setting.
- November 2008: Transitioned to a simplified single-tablet regimen of tenofovir disoproxil fumarate/emtricitabine (FTC)/efavirenz.
- January 2016: Switched to abacavir/3TC/dolutegravir (DTG) after osteoporosis diagnosis.
- September 2020: Switched to bictegravir (BIC)/FTC/tenofovir alafenamide (TAF) due to CV concerns.
Recent tests show viral load (<50 cp/mL) with CD4+ T cell count >700 cells/mm3. Extant medical issues include CV and cancer risk, STIs, ongoing use of chemsex-related substances, and poor mental health.
How Typical is This Patient Case and the Medical Needs in Your Clinical Practice Experience?
Lacombe confirmed that the largest proportion of patients seen in her clinical practice, around 50–60%, are older than 50 years, like Pepe. “Patients that have been living with HIV for many years and have been exposed to a wide variety of antiretroviral agents,” she noted. “And because they’re getting older, they have developed a range of comorbidities: CV comorbidities, neurological disorders, and a high prevalence of mental health disorders, mainly depression.”
What are the Main Challenges That Clinicians Face in Managing Older People with HIV Who Have Additional Comorbidities?
“I spend more time in the outpatient clinic talking about diabetes, cardiovascular disease, hypertension, obesity, weight gain, etc., than HIV control or CD4+ count,” noted Lacombe.
This point was echoed by Gandhi: “A big challenge in taking care of people with HIV is comorbidities. They have an accelerated ageing phenotype, so we want to be extremely vigilant in controlling cardiovascular risk factors.”
CV disease (CVD) is a particularly prevalent comorbidity in older people living with HIV. People living with HIV experience higher rates of CVD than the general population, yet traditional CV risk calculators do not account for HIV-specific factors and so may underestimate this risk.1,2 Drugs associated with chemsex may further increase the risk of CV events by elevating heart rate and blood pressure.3,4 Chemsex itself refers to the use of drugs, particularly psychoactive and disinhibiting substances (e.g., methamphetamine, cocaine, and gamma-hydroxybutyrate), in the context of sexual intercourse. Estimates indicate that rates of chemsex in Europe range between approximately 10–30%.5
Updated 2025 EACS guidelines on CVD prevention recommend assessing CVD risk over the next 10 years in people living with HIV, and considering ART modification if ≥10%.6 It is therefore important for clinicians to be aware of the available evidence for different ART regimens. In the TANGO Phase 3 open-label study, the switch to DTG/3TC improved most lipid parameters versus maintaining a TAF-based regimen through Week 144.7 Similarly, in a separate meta-analysis of 65 studies including 39,713 people living with HIV, significant increases in lipid profiles were seen 12 months after TAF initiation across all cholesterol types.8 In this analysis, 41.5% of patients switched from tenofovir disoproxil fumarate to TAF, which may have contributed to the change in lipid levels observed.8 In contrast, in the SOLAR RCT and the SCOLTA and SCohoLART real-world studies, cabotegravir (CAB) plus rilpivirine (RPV) LA was shown to have a neutral impact on lipids.9-12 Patients switched to CAB+RPV LA experienced no significant change in key lipid parameters and actually showed a potential reduction in the risk of developing metabolic syndrome.9-12
EACS guidelines on CVD prevention additionally recommend the identification and treatment of key modifiable risk factors, namely blood pressure, coagulation, glucose, and lipid levels.6 The recent Phase III REPRIEVE study investigated the effect of the lipid-lowering statin pitavastatin on major CV events in people with HIV. Pitavastatin was shown to reduce the incidence of major adverse CV events by approximately 35% versus placebo in people living with HIV on ART with low-to-moderate CVD risk and normal LDL range.13
The management of patient comorbidities forms an integral part of holistic HIV care, particularly in older people living with HIV who may face additional psychosocial challenges, Lacombe concluded. It should encompass treatment and prevention of medical comorbidities, most notably CVD, but also capture wider elements of the patient profile such as mental health issues, chemsex practices, and overall lifestyle.
What is the Prevalence of Mental Health Issues in the HIV Community, and How May it Present Itself in the Clinic, i.e., Reduced Adherence?
“The mental health of people living with HIV is absolutely paramount,” Lacombe stressed. “It’s a huge issue because there is such a burden due to stigmatisation because of the infection and discrimination associated with the HIV status.”
Lacombe acknowledged that the prevalence of mental health issues in HIV, especially depression, is “very high” but has been overlooked in the past. “Now that infection control is easier with effective ART, mental health issues are coming to the forefront,” she explained.
Recognising the signs of mental health disorders is critical to effective HIV care, particularly given emerging evidence linking mental health conditions to poor ART adherence.14,15 Guidelines recommend that patients with mental health needs should be provided with support to access specialist mental health care within a shared decision-making framework.6,16
Lacombe similarly recommends that clinicians adopt a holistic approach in the practice setting, asking patients general questions about their everyday lives, relationships, family, friends, and employment in order to assess their mental health. “I would encourage young healthcare professionals (HCP) to start the consultation with questions and exchanges,” she advised. “Questions which are not directly related to the control of infection but address the world environment of the patients.”
Pepe has never had established virological failure, but has experienced a number of virological rebounds, which may be related to gaps in adherence to oral ART. No emerging resistance was evident on the latest genotypic test.
How Do We Differentiate Between Blips and Low-Level Viraemia?
The origins of viral blips are complex and multifactorial, and include high viral load and low CD4+ count, residual viraemia, assay variability, and external factors such as infections.17 Poor adherence or suboptimal drug absorption can also cause transient drops in ART levels, leading to brief viral replication.17 Viral blips and low-level viraemia (LLV) are important in HIV because they can signal increased risks of virological failure and the emergence of drug resistance. Misdiagnosing LLV as an error rather than a potential sign of issues like ART non-adherence can also prevent clinicians from addressing the root cause, potentially leading to repeated or worsening problems.
Pooled post-hoc analysis of the FLAIR, ATLAS, ATLAS-2M, and SOLAR RCTs showed a similarly low frequency of blips and LLV with CAB+RPV LA as with oral ART18 (Figure 1). Similar data were observed in the ATHENA cohort, with only 1/588 patients receiving CAB+RPV LA experiencing a viral blip (versus 0/1,005 in the oral ART arm).19 This is notable, as ATHENA was a real-world study where viral load in the LA arm was monitored more regularly versus oral standard-of-care.
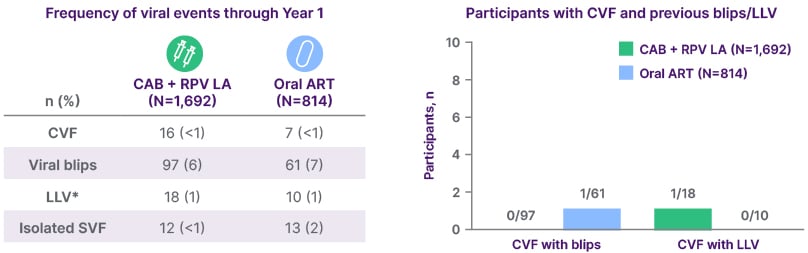
Figure 1: Pooled post-hoc analysis of FLAIR, ATLAS, ATLAS-2M, and SOLAR trials.18
*≥2 consecutive VLs 50–<200 c/mL.
CAB: cabotegravir; CVF, confirmed virological failure; LA: long-acting; LLV: low-level viraemia;
RPV: rilpivirine; SVF, suspected virological failure; VL, viral load.
Importantly, in further real-world data, where patients may have been monitored more regularly, viral blips and LLV were not found to be associated with virological failure among patients receiving CAB+RPV LA.20,21 An expert consensus definition of virological failure for CAB+RPV LA has recently been developed.22
Summary
Overall, when evaluating a patient like Pepe for potential LA therapy, it is important to consider all of the above factors and more:
- Ongoing mental health needs.
- Use of chemsex and potential drug–drug interactions.
- Improving adherence (as chronic viraemia can impact CVD risk).
- Polypharmacy and comorbidities.
- Optimisation of CVD risk factors (e.g., weight, lipid management).
To comply with the product label, viral load must be undetectable with no history of virological failure prior to initiating LA CAB/RPV.
CASE STUDY 2: ADHERENCE – SUPPORTING AN OLD PROBLEM WITH A NEW SOLUTION
The case of BA, a 43-year-old, single, cisgender man who has sex with men, was presented by Antoine Faycal, Charles-Foix University Hospitals, Paris, France. Originally from the Ivory Coast, BA has been living in France since 2011, when he was first diagnosed with HIV-1. This patient has faced social issues such as unstable housing and has had difficulties accepting his diagnosis. He is an active smoker and episodic binge drinker, and also has concomitant depression (treated with sertraline).
The patient has subtype C HIV, with a nadir CD4+ T cell count of 118 cells/mm3 and a zenith viral load of 95,000 copies/mL. Hepatitis B has been successfully treated and the patient is RNA negative. No opportunistic infections have been reported.
The treatment history indicates multiple adherence problems, and the patient has never been entirely suppressed. In April 2022, BA was switched to TAF/FTC/darunavir/cobicistat; however, the pattern of non-adherence and low engagement with care continued. Viral load was persistently 330–9,500 c/mL, with a stable CD4+ T cell count of approximately 200 cells/mm3. No emerging resistance was seen with historic or most recent genotypic testing.
Given the clear challenges facing this patient with adherence to oral ART, consideration was given to placing him on LA injectable therapy.
How Does This Case Reflect the Challenges of Non-Adherence Seen in Clinical Practice?
Gandhi agreed that this case study was reflective of patients seen in her everyday clinical practice who face challenges in adherence to daily oral ART. “Every clinical setting has adherence challenges such as substance use, addiction, food insecurity, housing insecurity, and mental health concerns,” she explained.
During the ViiV-sponsored symposium, the audience was asked what percentage of their own patients struggle with adherence, and the majority of poll respondents said one in every eight. However, in the PP2 study, an anonymous web-based survey of adults living with HIV from 25 countries, on average, one in four of the 2,389 patients surveyed (24%) reported missing an ART dose five times or more within the past month; this suboptimal adherence varied between 10–62% across the different subpopulations23 (Figure 2). These results suggest that suboptimal adherence may be more common among people living with HIV than is observed in clinical practice, as visits every 6 months with viral load monitoring may fail to account for the doses that may be missed in between. Suboptimal adherence was also associated with lower self-reporting of optimal physical, mental, sexual, and overall health among people living with HIV.23
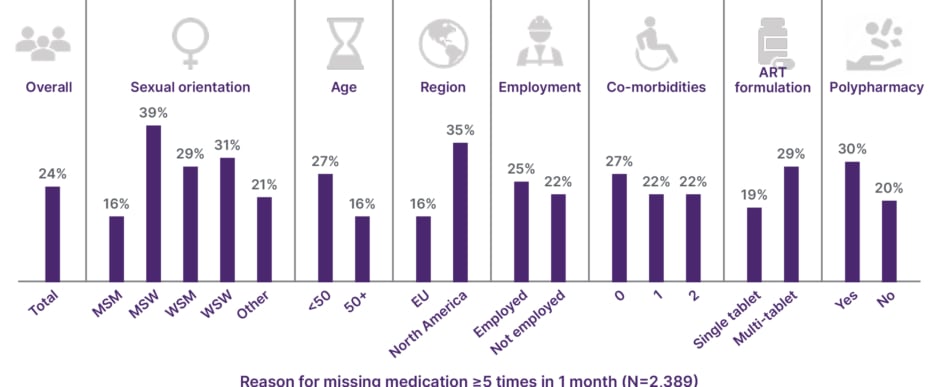
Figure 2: Positive Perspectives 2: suboptimal adherence is common among people living with HIV.
ART: antiretroviral therapy; MSM: men who have sex with men; MSW: men who have sex with women; WSM: women who have sex with men; WSW: women who have sex with women.
Gandhi noted that “both external and internal stigmas can make it hard to maintain medications,” thereby contributing to non-adherence to oral ART.
Overall, these clear challenges in taking daily medication underscore the importance of ART strategies and other interventions, such as LA injectables, to support medication taking and improve adherence, she emphasised.
How Can Clinicians Better Support Adherence?
Addressing adherence should form part of an integrated package of support, with clinicians deploying a variety of recommended compliance and retention strategies.24 “A number of adherence interventions have been designed and can be particular to the setting,” Gandhi elaborated. “For example, adherence clubs, peer counselling, and two-way text-messaging can improve adherence. There is also an intervention in which drug level feedback is provided to the patient (preferably in real time using point-of-care adherence metrics), which can improve adherence.” It is important to bear in mind that reduced adherence can be momentary, periodic, cyclical or long-term depending on the underlying drivers.
“Also, we always want to explore what can make it difficult to take oral ART every day,” she continued. These questions should be asked without judgement. “Ask patients about addiction, their housing situation, food security, depression, anxiety, other mental health conditions, and concomitant medical illnesses.”
Lacombe also stressed the importance of addressing pill fatigue and ensuring patients are aware of advances in therapy like LA injectables and how these could fit into their everyday lives and schedule. “I talk about innovations in treatment at every outpatient clinic because you want patients to benefit from access to innovation,” she stressed. All eligible patients with adherence issues should therefore receive this education around each of the options available to them for treatment.
What Are the Future Care Options for Patients Who Face Challenges with Adherence to Oral Antiretroviral Therapy?
“The most incredible advance we’ve had over the last 4 years is the advent of LA ART,” remarked Gandhi. “It has been extremely exciting and gratifying to see how beneficial these drugs have been for those with adherence challenges.”
“The combination of long-acting CAB+ RPV in our clinic, and in multiple clinical settings around the world, has really improved virological suppression rates in those living with challenges to taking oral ART,” she added.
As Gandhi outlined, increasing evidence is being amassed to support the effectiveness of LA therapy in people with HIV who have difficulty adhering to ART, including data from clinical trials and retrospective real-world studies.25-32 Collectively, these studies “have shown that LA injectable therapy was superior to oral ART in those with adherence challenges,” she noted, “And we have also found the same thing in our clinic: higher rates of virological suppression with the use of LA CAB+RPV.”
Another tool in the LA armoury is the HIV-1 capsid inhibitor, lenacapavir, which is indicated for multidrug-resistant HIV-1 infection in adults in whom it is not possible to construct a suppressive antiretroviral regimen.33 In the Phase III CAPELLA study, lenacapavir demonstrated a greater reduction in viral load (VL) from baseline (88%) versus placebo (17%).34 In a further Phase II study, most patients maintained virological suppression at Week 24 after switching to weekly oral islatravir+lenacapavir from daily BIC/FTC/TAF,35 leading to Phase III studies of this combination being launched.
Gandhi explained that LA therapies can help relieve some of the stigma that contributes to non-adherence to oral ART, as supported by data from the SOLAR and MOCHA clinical studies of CAB/RPV.36,37 “Stigma was relieved by using the LA CAB+RPV and not having to take pills home, but instead coming into a centre and getting the injection,” she elaborated.
Lacombe agreed: “When patients have barriers to oral therapies, for example, stigma and worries around forgetting pills, then the benefit of LA is it leads to a very high improvement in treatment satisfaction.”
Whereas for the past several decades the only option has been oral therapy, LA injectables may help incentivise those patients with adherence challenges to come to the clinic to explore new treatment options. Looking ahead, future LA treatments on the horizon will also offer a broader choice of options to people with issues adhering to daily oral ART, Gandhi concluded.
After 4 weeks on oral ART to achieve virological suppression, BA was switched to CAB+RPV every 2 months, and viral load remained undetectable after 6 months of treatment.
What Would You Do in Case of a Late-show for an Injection Appointment, and How Should This Be Managed?
Encouragingly, high rates of adherence to CAB+RPV LA (e.g., on-time injections) have been observed in Phase IIb/III RCTs and real-world evidence in people who have difficulty adhering to oral ART, with between 94–98% of patients receiving their injections on time.26,27,29,31 In cases where patients do miss their CAB+RPV LA injection, this should be managed with oral bridging. If a patient plans to miss a scheduled injection visit by more than 7 days, daily oral tablets can be used for up to 2 consecutive months, Gandhi advised.38-40
“For those who are on LA CAB plus RPV, we see them essentially every injection visit,” Gandhi elaborated. “We will have a touch point with the patient because we are administering those medications in the clinic. So will check in every 2 months: that’s six times a year.”
Summary
BA provides a real-world case study example of a person living with HIV who could benefit from LA treatment options:
- Poor adherence and difficulty engaging with care.
- Adherence can be incentivised with LA injectable therapies.
- The risk of HIV transmission is reduced with LA options.
- LAs can support regular touchpoints to support patients’ unmet needs.
A multidisciplinary team and shared decision-making remain important for long-term care management.
CASE STUDY 3: MORE THAN IN WOMEN, IN HIV CARE
Kirsty Abu-Rajab, NHS Forth Valley, Scotland, UK presented the third case study of a 21-year-old female from Edinburgh, UK, who was newly diagnosed with HIV in routine STI screening and has opted not to share her HIV status. She lives alone, works in an administrative role, is currently single, and has no children.
Baseline tests revealed HIV subtype C with a viral load of 127,000 copies/mL, a CD4+ T cell count of 556 cells/mm3, and no resistance-associated mutations. Hepatitis B core antibody and HBsAb were negative. The patient’s baseline BMI was 29.8 kg/m2 (weight: 84.0 kg; height: 168 cm).
What are the Options for a Treatment-Naïve Person Newly Diagnosed with HIV?
This case study typifies some of the issues that female patients living with HIV, who comprise a substantial proportion of the overall population, may experience, Orkin confirmed. Although the global incidence of HIV is declining, it remains high among adolescent girls, young women, and females >50 years.41 The ART options currently recommended first-line by EACS have demonstrated efficacy across Phase III, double-blind RCTs;6 however, no currently available LA is recommended/licensed for use in treatment-naïve patients.
Orkin outlined how an early switch to CAB+RPV after induction with oral DTG/3TC was evaluated in the VOLITION Phase 3b, non-randomised, open-label study. In total, 89% of 171 participants chose to switch to CAB+RPV LA, citing advantages such as not worrying about missed doses, convenience, and a lack of pills. Patients also valued being empowered with the option to switch to a treatment that better matched their lifestyle.42
The patient from Edinburgh, UK, was started on BIC/FTC/TAF and achieved suppression. However, after 18 months, she expressed concern about weight gain, which had increased by >5% from 84.0 kg (overweight) to 90.2 kg (obese) since starting treatment.
How Big an Issue is Weight Gain on Oral Antiretroviral Therapy in Your Clinical Experience?
“Weight gain is an issue for people with HIV and for everyone in society,” Orkin commented. “Some old drugs were weight attenuating, but then people gained weight when moved to the newer, more tolerable regimens.”
Orkin explained that initiating ART in treatment-naïve people with HIV can also induce a ‘return-to-health’ effect on weight, so it is important to differentiate this from undesirable and/or excessive weight gain caused directly by ART.43,44 Major HIV guidelines confirm that certain drug classes, notably integrase strand transfer inhibitors and TAF, are associated with greater weight gain than other ARTs.6,45-47 In contrast, in the SOLAR RCT and the SCOLTA and SCohoLART real-world studies, CAB+RPV LA was not associated with any significant changes in patients’ weight.9-12
Updated EACS guidelines state not to switch ART solely on the basis of weight gain as an adverse event.6 In the audience poll conducted during the symposium, the majority of delegates who voted (63%; N=81) said they would recommend exercise and a healthy diet in a patient reporting weight gain.
During subsequent discussions around treatment, this patient proactively enquired about CAB+RPV LA.
How Does Treatment Preference and Shared-Decision-Making Impact Outcomes?
Orkin asserted that supporting patients’ decisions around treatment was important, but within the confines of the clinical evidence and good practice. “Shared decision-making is about communicating the science and the guideline recommendations so people are fully informed,” she explained. “A recently published […] expert opinion piece covers a lot of hot topics, such as BMI, hepatitis B, pregnancy, etc., so clinicians can read more details in this publication,” Orkin added.48
The Positive Perspectives 3 study has shown that shared decision-making and involvement with care are associated with patient satisfaction and QoL. In this survey of nearly 700 adults living with HIV from 16 countries, 75.4% of respondents felt involved in their care decisions, and 60.3% stated that their ART regimen was jointly decided with their HCP.49 The Ask Us study provides further perspectives on treatment optimisation discussions between patients and HCPs across Europe. HCPs of people who have CAB+RPV LA were more likely to have brought it up with their patients, versus HCPs of those who had never received CAB+RPV LA, suggesting that raising LA options with patients influences uptake of LA CAB+RPV.50
What are Some of the Main Challenges That Clinicians Face in Managing Female Patients with HIV?
Orkin summarised some of the main educational needs for clinicians when managing patients with potential reproductive needs. “Firstly, does the person wish to conceive? If so, it’s important to understand regimens that may or may not be suitable during pregnancy,” she noted. If a patient does not want to conceive, then contraception should be discussed. For patients undergoing menopause treatment, it is important to assess potential drug interactions with HIV therapy, Orkin explained, and similarly “for people on hormones for other reasons, such as gender affirming hormone therapy.”
Few ARTs are fully licensed for use during pregnancy due to insufficient data; however, guidelines nonetheless recommend initiation or continuation of ART in pregnant women with HIV. In ART-naïve pregnant women, the preferred regimen according to updated EACS 2025 guidelines is two nucleoside reverse transcriptase inhibitors plus an integrase strand transfer inhibitor.6
“There are certain regimens that are recommended as options in pregnancy and some that are not,” Orkin concluded. “So, it’s important to follow the guidelines in the country in which you live and discuss whether the current regimen may need to be switched.”
Summary
This case study highlights some key considerations for clinicians when caring for a newly diagnosed female patient with HIV:
- Data on weight gain can be conflicting.
- Switching to LA injectable once virologically suppressed may offer incentives to remain suppressed.
- The risk of hepatitis B reactivation is low but should be monitored.
- Shared decision-making is important for longer-term optimisation of adherence and outcomes.
- Women need to understand the options and the risks of ART in pregnancy and should be given the choice as part of shared decision-making.
UNMET NEEDS AND ROLE OF LONG-ACTING THERAPY
Finally, from a broader perspective, experts considered how LA injectable therapy has helped to address unmet needs in HIV care.
“People want therapies that remove the need for daily good decision-making and give the freedom to receive 6 treatment days a year instead of 365,” Orkin emphasised. “CAB+RPV is the first ever complete LA regimen for people with viral suppression….it’s a new paradigm.”
“We have had incredible success with the use of long-acting CAB+RPV,” Gandhi confirmed. “Both in those patients who want the convenience of every-2-month injectable administration, and for those with adherence challenges.”
“This is a great opportunity for patients to decrease the pill burden,” Lacombe reiterated, “to reduce drug–drug interactions, and to enhance adherence.”
Orkin concluded: “With LA therapy, people also talk about normalcy, going back to a pre-diagnosis state in terms of how they feel about themselves, and a more positive outlook generally.”
CONCLUSION
LA injectables have assumed an important position in today’s HIV treatment landscape, and can offer patients with a variety of unmet medical needs improved choice around treatment. Among HCPs, a level of cautiousness with any new treatment approach is understandable; however, LA therapy is supported by robust clinical data and is included in updated EACS guidelines. The benefits of LA injectables include improved adherence, reduced drug–drug interactions, neutral metabolic impact, and improved patient QoL. Clinicians should therefore consider LA options during multidisciplinary team discussions and in shared decision-making with patients in order to optimise HIV care moving forward.
| Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441. If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market here: https://www.gsk.com/en-gb/contact-us/report-a-possible-side-effect/. |
Indication
Cabotegravir (Vocabria) is indicated, in combination with rilpivirine injection, for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and adolescents (at least 12 years of age and weighing at least 35 kg), who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with agents of the non-nucleoside reverse transcriptase inhibitor (NNRTI) and integrase inhibitor (INI) class.
Rilpivirine (Rekambys) is indicated, in combination with cabotegravir injection, for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and adolescents (at least 12 years of age and weighing at least 35 kg) who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with, agents of the non-nucleoside reverse transcriptase inhibitor (NNRTI) and integrase inhibitor (INI) class.
Dovato (dolutegravir/lamivudine) is indicated for the treatment of HIV-1 in adults & adolescents above 12 years of age weighing >40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.
NX-GBL-HVX-JRNA-250001 | November 2025







