Meeting Summary
Despite substantial advances in the HIV arena over the past few decades, the world is not currently on track to succeed in reaching the Joint United Nations Programme on HIV/AIDS (UNAIDS) target of ending AIDS as a global health threat by 2030. During this ViiV-sponsored symposium at the European AIDS Clinical Society (EACS) Conference 2025, a panel of leading infectious disease experts engaged in a masterclass debate on the current state of the HIV epidemic and progress made towards the goal of eradicating it. Experts explored the potential for changing the trajectory of the HIV epidemic with decisive action and assessed how long-acting (LA) injectable therapies may help to achieve this objective through effective prevention and treatment.
Where Are We Now?
Karine Lacombe, Professor at Sorbonne Université, Paris, France, set the scene by outlining the current trajectory of the HIV epidemic and progress made towards achieving key UNAIDS targets. In 2014, UNAIDS launched a strategic framework to accelerate efforts to end the HIV epidemic by 2030.1 Targets for 2025 were for 95% of people living with HIV to know their HIV status, 95% of those who know their status to be on treatment, and 95% on treatment to be virally suppressed.2 According to the latest UNAIDS Global AIDS Update for 2025, these 95% goals have been met by 87%, 89%, and 94% of people, respectively.1 An additional fourth target has also been proposed for 90% of patients who are virally suppressed to have a good health-related quality of life.3 However, as Lacombe pointed out, even if all these four key targets were met, they would not result in an HIV-free generation, nor the end of the epidemic.
The Executive Director of UNAIDS has acknowledged that the world is not currently on track to reach the target of ending AIDS as a global health threat by 2030. This mission was to reduce new HIV infections to under 370,000 by 2025 and achieve zero new cases by 2030. However, in 2024, there were an estimated 1.3 million new HIV infections worldwide, which was over three times the 2025 target.4 Notably, over half of those diagnosed in the WHO EU region in 2025 were identified at a late stage, and nearly a third had already progressed to an advanced stage of the disease.4
Worldwide, 40.8 million people were living with HIV in 2024, together with the approximately 1.3 million new diagnoses. Half of all people newly diagnosed with HIV in 2024 (approximately 650,000) were living in Eastern, Southern, Central, and Western Africa, while there were 300,000 new diagnoses in the Asia Pacific region.1 Focusing on Europe, there were an estimated approximately 2.3 million people living with HIV in the wider European region in 2023, with the majority (62%) located in Eastern Europe, followed by Western (36%) and Central Europe (2%).5 Significant increases in the number of new HIV diagnoses were also seen in England, France, Spain, and Italy in the wake of the COVID-19 pandemic.6-9
In Europe, as in the wider world, the issue of late diagnosis remains pertinent, particularly as it delays the initiation of antiretroviral therapy (ART). Over 50% of new HIV cases in the WHO European Region in 2022 were diagnosed late in people with a cluster of differentiation (CD)4+ cell count <350 per mm3. Late diagnosis was particularly common in those aged over 50 years, where over 65% of cases were diagnosed late, and in men and women contracting the disease via heterosexual transmission routes (>50% late diagnoses in both groups).10
Over the next 5 years, the estimated prevalence of people living with HIV is forecast to increase, driven by new infections and people living longer with HIV. Across France, Spain, Italy, Germany, and Ireland, these rises will result in around 670,318 people living with HIV by 2030. Lacombe emphasised the projected costs associated with ‘complacency’ around increasing HIV prevalence and the financial burden of caring for both current and new cases. The annual cost of this complacency can be calculated as the number of people living with HIV, multiplied by the most recent healthcare system cost per person per year. Using this approach, estimated costs of care between 2025–2030 are expected to increase substantially, totalling 21.68 billion EUR in France, 9.12 billion EUR in Spain, 11.97 billion EUR in Italy, 13.24 billion EUR in Germany, and 694.47 million EUR in Ireland. This equates to a total expenditure on HIV care of 56.7 billion EUR across these five European countries over the next 5 years.11
Reported diagnoses provide an important measure of new infections over time. As Lacombe explained, the projected numbers of people becoming newly diagnosed with HIV, using data from the European Centre for Disease Prevention and Control (ECDC), shows a concerning recent uptick in diagnoses.5 In an attempt to gain insight into future trends, three linear models over different timeframes were used to project the trajectory of new HIV diagnoses across eight European countries, and calculate the additional cost burden created by these new cases. Based on the 2019–2023 projection, the UK, Poland, and Ireland show the steepest future trajectory in diagnoses; however, increases are still projected in other countries. Notably, no country is on track to reach zero new diagnoses by 2030 based on any projections. This highlights the importance of intervention strategies to prevent the steeper trajectories from being realised, Lacombe stressed.11
In summary, Europe stands at a critical juncture and inaction will incur a high cost, Lacombe cautioned. Across the continent, 24 out of 26 countries failed to meet the goal of diagnosing 95% of people living with HIV. Progress in reducing HIV transmission is stalling and diagnoses are rising in some regions, which means that, without renewed effort, the 2030 UNAIDS targets will undoubtedly be missed.11
Are We Equipped to End the Epidemic?
Barriers and Solutions
Christoph Spinner, Professor at the Technical University of Munich Hospital, Germany, outlined the barriers that currently exist within HIV and provided further insights into the obstacles that continue to stand in the way of optimal care (Figure 1).11
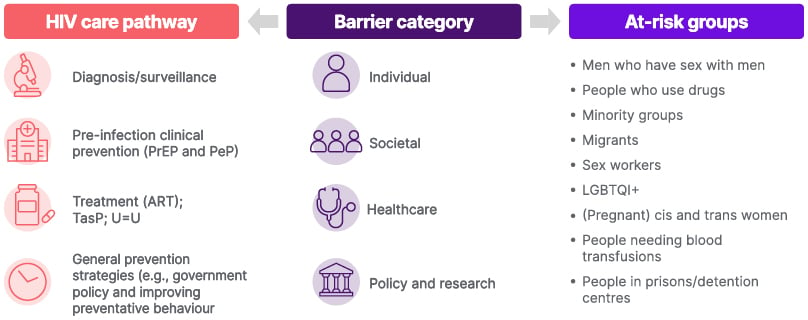
Figure 1: Barriers framework.11
ART: antiretroviral therapy; cis: cisgender; LGBTQI+: Lesbian, Gay, Bisexual, Transgender, Queer or Questioning, Intersex, Plus; PeP: post-exposure prophylaxis; PrEP: pre-exposure prophylaxis; TasP: treatment as prevention; trans: transgender; U=U: undetectable = untransmittable.
Stigma is a key societal barrier prominent in the literature and spanning across the barrier categories. Stigma continues to have a wide-reaching impact on people living with HIV, as well as on the rate of new diagnoses.11 On an individual level, stigma can inhibit access to HIV testing and care, and discourage adherence to medication due to fear of disclosure.11 Systemically, it can influence the quality of care received by people living with HIV due to biases among healthcare professionals, and socially, it can lead to isolation created by a fear of rejection.11 Data on the issue of stigma are also hard to quantify and measure, which adds an additional level of complexity to strategies for addressing it. There is also limited community-based involvement in helping mitigate barriers to accessing HIV education, prevention, and treatment within specific communities. Limited knowledge and awareness of HIV, including low perceived risk and lack of awareness of available services to address concerns, represents another key barrier, which is compounded in some populations by limited use of prevention strategies outside of medical intervention.11
Continuing the debate, Jonathan Schapiro, Professor at Sheba Medical Center, Tel Aviv, Israel, proposed that the key questions that are crucial to ending the HIV epidemic have already been answered in the affirmative. We have clearly defined our objective, which is to transform HIV into an easily manageable, rare, occasional infection, said Schapiro, and we are motivated because we enjoy life less when other people suffer. We have the tools needed to succeed, benefiting from over 30 years of drug development in the field, and funding can be secured if this is a high enough public health priority. We also have a plan, Schapiro concluded, “let’s see what has been done and work on it together.”
In practice, multiple individuals, as well as societal, healthcare, policy, and research barriers, continue to limit efforts to eliminate HIV transmission in Europe.11 This important topic was addressed in a recent report by the Office of Health Economics (OHE), which examined the ongoing HIV epidemic in Europe, highlighted key barriers to progress towards the UNAIDS’ 2030 targets and potential solutions, and explored the ‘cost of complacency’ of continuing on the current trajectory. To develop this report, the OHE conducted targeted literature reviews to explore the barriers to progress, solutions, and related costs. To validate and refine findings, two rounds of interviews were held, first with expert stakeholders, including clinicians and policymakers, and then with patient advocates. Additionally, a roundtable was convened with experts to prioritise key barriers and solutions, and two subsequent meetings were held with healthcare professionals to validate projected trends, cost analyses, and the quantitative impact of proposed solutions.11
As Spinner explained, a comprehensive, multi-faceted policy approach that simultaneously targets prevention, screening, and treatment is therefore needed to meet the ambitious goal of ending the HIV epidemic. The most high-impact barriers to eliminating HIV transmission can be identified by assessing their magnitude of negative impact and the ease with which they can be addressed. This two-way approach was undertaken by a panel of experts as part of the OHE report, with all barriers and solutions ranked on a heatmap looking at the magnitude of impact and ease of addressing/implementing.11
Using this approach, diversification of treatment options and testing outside the clinic were identified as two of the most impactful and feasible solutions.11 As Spinner explained, by highlighting where the most impactful issues meet the most feasible solutions, decision-makers can prioritise addressing the most pressing challenges using the most achievable solutions to ultimately overcome barriers to eliminating HIV transmission. For example, encouraging governments to look beyond medicalised settings to offer diagnosis and treatment, e.g., offering pre-exposure prophylaxis (PrEP) through community-based organisations, would increase uptake in populations with disproportionately low access to healthcare services. Similarly, diversification of treatment options for PrEP and ART and increased use of LA therapies could help to simplify the treatment regimen and thereby reduce the burden on people living with HIV.11
Spinner also outlined additional proactive steps towards ending the HIV epidemic, such as expanding availability and access to a variety of HIV testing methods, increasing collaboration with communities, and the establishment of dedicated data-sharing platforms. It is also important to promote public campaigns and community-specific outreach programmes to inform and reduce misconceptions around HIV.11 As emphasised in the OHE report, partnering with community organisations to develop and deliver tailored and targeted education and support programmes for underserved groups is essential in order to ensure equity of access to resources and services.11 Such organisations are often better positioned to engage diverse communities which can be out of reach of healthcare systems and services.11
Scope for Improvement
HIV incidence remains high in key populations despite advancements in treatment and prevention, making epidemic control by 2030 unlikely without major strategy changes.12 Oral PrEP is effective but underutilised, with low uptake and adherence, particularly in low and middle-income countries.12 Current strategies also miss many at-risk individuals, highlighting the need for broader, more inclusive prevention approaches.12 For example, patients with disabilities face additional challenges accessing HIV services and treatment across the care continuum, from HIV prevention through to lifelong therapy and support after testing positive.13 On the treatment front, LA injectables and broadly neutralising antibodies show promise in helping to address the HIV epidemic, but are associated with challenges such as high costs, coverage, and implementation.12 Finally, a preventive HIV vaccine remains essential for population-level impact, but development is still ongoing and unlikely to result in a licensed product for many years.12
PrEP is an important biomedical prevention strategy for people at risk of HIV acquisition that can significantly reduce HIV infections in at-risk groups, including men who have sex with men (MSM), transgender women, and people who inject drugs. Approximately 6.2 million people had initiated oral PrEP at least once by the end of 2023, although patterns of use vary globally.14,15 In the WHO European region, there were an estimated 285,000 people on PrEP in 2023.16 At current levels of uptake, Europe will therefore fall short of meeting its target of 500,000 people on PrEP by 2025, driven in part by difficulties in reaching all vulnerable populations with oral prophylaxis.17 Data collected across Europe show that key populations currently missing out on PrEP include migrants (both documented and undocumented), sex workers, prisoners, transgender people, people who inject drugs, gay men, and other MSM.18
Addressing Adherence Challenges
Another important challenge is suboptimal adherence to oral ART, which is common in real-world practice as evidenced by the Positive Perspectives study of 2,389 people living with HIV across 25 countries. It found suboptimal adherence in 24% of participants, with 71% having missed ART ≥1 time for any reason in the past month.19 The recent GLACIER study identified the top reason for not taking daily oral ART as patients simply forgetting (data on file). Other reasons for non-adherence included running out of medication, impact on social plans, and side effect avoidance (data on file).
In total, an estimated 38% of people with HIV have suboptimal adherence to oral ART due to factors such as pill fatigue, emotional challenges, stigma, busy lifestyles, and the complexity of dosing regimens.20-22 This non-adherence is associated with a lower CD4 count and an increased risk of transmission and resistance, contributing to higher healthcare costs and increased AIDS-related mortality.20,23 To combat non-adherence, EACS guidelines emphasise the importance of regular assessment of barriers faced by people with HIV to ensure tailored care to meet their needs and preferences.24 LA injectables like cabotegravir (CAB) plus rilpivirine (RPV) can also help to overcome some adherence challenges, as this directly-observed therapy removes the need for compliance to daily dosing while allowing healthcare professionals to track doses received.25
Retention and engagement in care are critical for improving outcomes at both the individual and population levels in HIV, but are often suboptimal due to factors such as stigma, low treatment satisfaction, and psychological challenges.26-32 Given the strong link with virologic suppression, high retention in care was shown to be associated with improved survival, decreased HIV-related complications, and reduced HIV transmission.28-30 Conversely, poor engagement was associated with numerous negative outcomes, including increased hospitalisation rates, increased HIV transmission, and reduced survival.29,32 Practical ways to address this important barrier of stigma include solutions such as public awareness campaigns and educational initiatives. As explained in the OHE report, educational public awareness campaigns that are co-created with affected communities, use people-first language, and are targeted at both general populations and healthcare professionals, have the potential to most effectively combat prejudice and stigma.11
The Role of Long-Acting Therapy in Prophylaxis and Treatment
Various PrEP options are currently reflected in international clinical guidelines, but recommendations at the national level can vary from country to country. Several oral PrEP regimens are available, including tenofovir disoproxil fumarate/emtricitabine (FTC) and tenofovir alafenamide/FTC, indicated as a daily regimen for populations at risk of HIV acquisition and MSM/transgender women, respectively.24,33,34 Tenofovir disoproxil fumarate/FTC is also used off-label as on-demand event-driven oral PrEP, while dapivirine is indicated for cisgender women only as part of combination prevention approaches.24,33-36
In addition to these established daily oral PrEP regimens, LA PrEP options are now also available. These include CAB LA, which is indicated for all populations at risk of HIV acquisition and is administered via intramuscular injection every 2 months.33-35 Lenacapavir, given via subcutaneous injection every 6 months, is another LA PrEP option for populations at risk of HIV acquisition.37,38
Spinner suggested that the beneficial impact of LA injectable PrEP on HIV incidence may have the potential to change the trajectory of the epidemic. Data modelling the impact of rapid LA injectable PrEP scale up in MSM in the Netherlands demonstrated the potential to avert between 14–21% of new infections within 10 years of introduction.39 Similar USA modelling studies have projected annual reductions in HIV incidence between 4.3–36.3% with the use of LA injectable PrEP for MSM.11,39
Importantly, high directly observed adherence has been seen in LA injectable therapies used for both PrEP and treatment in RCTs and real-world studies. Adherence, evaluated by different measures, ranged from 91.5–93.0% for CAB PrEP and from 88.5–97.0% for CAB+RPV LA as treatment.40-48 Similarly, adherence to lenacapavir was 93–94% when used as PrEP and 98% as treatment.49-51
This improved adherence with CAB+RPV LA over daily oral ART is supported by real-world evidence from several studies showing maintenance of virological suppression in up to 98% of patients with a history of adherence challenges treated with CAB+RPV LA.46,52 In the USCD Owen Clinic study, 81% (n=44/54) of patients with a history of adherence challenges who received CAB+RPV LA maintained virologic suppression through Week 48.52 Similarly, in the JABS study, 97% of CAB+RPV-treated patients maintained virologic suppression at 12 months, of whom 27% had a history of adherence challenges.46
The importance of diversifying PrEP and ART options for treating HIV is reiterated in the OHE report. It notes that future ART formulations with LA modalities may offer important options that better align with people’s preferences and ultimately improve HIV prevention, adherence, and equity of access, thereby contributing towards the achievement of UNAIDS 95-95-95 targets.11
Conclusion
Three key pillars must be addressed to achieve UNAIDS targets moving forward and end the HIV epidemic, Lacombe concluded. Firstly, around awareness and prevention, it is important to increase recognition of HIV risk, boost PrEP uptake, and reduce complacency with oral medication. Secondly, it is important to focus on availability and access to care. This should encompass equal access to biomedical HIV treatment and prevention options (both oral and LA injectables), increased adoption of LA options and future innovations, and improved retention in care. Last but not least, it is vital to improve HIV diagnosis rates and move increasingly towards early disease detection and treatment.
Summary
The current state of the HIV epidemic signals a call to action to governments and healthcare systems. There is an urgent need to:
- Actively address barriers such as stigma with practical solutions.
- Improve adherence to antiretroviral treatment in people living with HIV.
- Harness the potential of LA therapy to tackle existing challenges in HIV care.
- Scale-up access to, and availability of, innovative solutions like PrEP and
LA injectables. - Take positive and proactive steps towards eradicating the HIV epidemic through effective prevention and treatment.
| Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441. If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market here: https://www.gsk.com/en-gb/contact-us/report-a-possible-side-effect/. |