Abstract
Growing evidence suggests that biological sex influences disease risk, clinical presentation, treatment response, and prognosis across neurological conditions. Despite this increasing awareness of important sex differences, neurological research and clinical care remain insufficiently tailored to females.
In this review, the authors highlight the importance of integrating sex-specific considerations into precision medicine for neurological disorders. Focusing on five high-prevalence and high-burden neurological conditions (epilepsy, migraine, stroke, multiple sclerosis, and neurodegenerative diseases), this review identifies critical knowledge gaps and actionable opportunities for advancing care for females. Such gaps and opportunities include: 1) improved pregnancy and lactation data in epilepsy; 2) hormonal influences across the menstrual cycle, pregnancy, and menopause in migraine; 3) sex-based disparities in symptom recognition, treatment access, and rehabilitation for stroke patients; 4) the influence of sex hormones on disease onset, progression, and prognosis in multiple sclerosis; and 5) sex differences in pathophysiology and clinical trajectories in neurodegenerative diseases.
This review proposes a roadmap for integrating sex-based considerations into three key domains: clinical care, research, and neurology training. Prioritising and advancing these initiatives is essential for improving neurological care and represents a critical step towards equitable precision medicine.
Key points
1. Sex-specific biological factors influence neurological disorders throughout life, impacting clinical presentation, response to treatment, and prognosis, yet they remain underexplored in neurology. As clinicians move towards tailored approaches to patient care with precision medicine, integrating sex-specific considerations will be essential.2. Sex-specific considerations for female patients include pregnancy-related safety data for anti-seizure medications, hormone-related influences on migraine disease severity, sex disparities in stroke care, sex-specific disease course in multiple sclerosis, and sex-based differences in incidence and presentation of neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease.
3. Addressing female-specific needs, especially during key life stages of pregnancy and menopause, ensures equitable representation in clinical trials, guidelines, and training programmes, and improves precision medicine in neurological care.
BACKGROUND
Historically, sex-related differences have been underexplored in neurology. It is important to highlight the distinction between sex and gender as, though different, they have previously been used interchangeably. Sex refers to biological and physiological attributes, such as chromosomal composition, hormonal profiles, and phenotypic traits that distinguish males, females, and intersex individuals. In contrast, gender relates to the socially and culturally constructed roles, behaviours, identities, and expectations associated with being a man, woman, or non-binary person. Sex and gender are independently important variables of both health and disease, interacting throughout an individual’s life course, and it is important for them to be differentiated in both research and the real-world setting.1 This review will focus on sex-based differences and their impact on neurological disorders.
Neuroscience research has been predominantly male-focused, a trend rooted in longstanding biases within scientific and cultural frameworks.2,3 This imbalance has significant implications for the quality and effectiveness of healthcare. In cardiology, the imbalance has been well documented. In the early 1990s, studies identified that female patients were less likely to be referred to diagnostic or interventional procedures when presenting with myocardial infarction, unstable angina, or chest pain, compared to male counterparts. In a cohort of patients hospitalised due to coronary heart disease in two different hospitals, 27.5% and 28.7% of males underwent angiography compared to only 16.1% and 17.7% of females, respectively.4 Similarly, with coronary revascularisation, 15.5% and 14.1% of males received treatment compared to only 7.4% and 6.5%, of females, respectively.4 This bias remains persistent, with a recent study showing that females presenting with chest pain are 20% less likely than males to have a troponin test performed, 36% less likely to be admitted to a specialised unit, and 35% more likely to die in the emergency department.5 Furthermore, although the prevalence of ischaemic heart disease tends to be higher in males within specific age groups, as females tend to live longer, in absolute numbers, ischaemic heart disease affects more female than male patients.6,7 Furthermore, symptoms more commonly seen in females, such as pain in the back, jaw, or neck,6 are still often described and taught as ‘atypical’ ischaemic cardiac symptoms.8,9
In neurology, there is now increasing attention paid to the impact of sex differences on the epidemiology, access to care, diagnosis, response to treatment, and prognosis of several neurological disorders.10 For female patients, unique, sex-specific biological considerations shape brain development and vulnerability to neurological disorders throughout life. While hormonal shifts play a role, factors like brain structure, immune function, and genetics also contribute to why neurological disorders affect females and males differently. In adolescence, steroid sex hormones modulate brain and behaviour to organise neural circuits for adult social and reproductive behaviours. Several brain MRI studies show sexual dimorphisms across puberty, such as the increase in amygdala volume in males only and increases in hippocampus volume in females only.11 In addition, important differences in mental health conditions emerge in adolescence, such as the prevalence of depression in females while a greater prevalence of conduct and addiction disorders are observed in males.12 During pregnancy, there is evidence of a remarkable period of hormonally driven neuroplasticity with a linear decrease of grey matter and total brain volume, with a partial rebound in the postpartum period,13 tied to the rise in progesterone and 17β-oestradiol. Besides the biological effects of pregnancy in the brain, this important window in life is linked to the increased likelihood of vascular disorders and a decreased likelihood of relapses in several autoimmune disorders, such as multiple sclerosis (MS), which have a higher risk of relapse postpartum, while in others, such as myasthenia gravis, 30–40% of patients have disease exacerbation.14,15 Finally, menopause, comprising up to 40% of a female’s lifespan, introduces cognitive and neurological shifts not explained solely by the decline of oestrogen. For example, postmenopausal females are at a higher risk for Alzheimer’s disease (AD), a condition that affects two-to-three times more females than males.16 Genetic risk factors like APOE-ε4 may impact females more strongly, possibly due to interactions of brain metabolism with ageing.16 Important life stages such as pregnancy and menopause represent key windows of opportunity to understand more precisely the impact of sex-specific biological factors on neurological illness, as summarised in Figure 1.
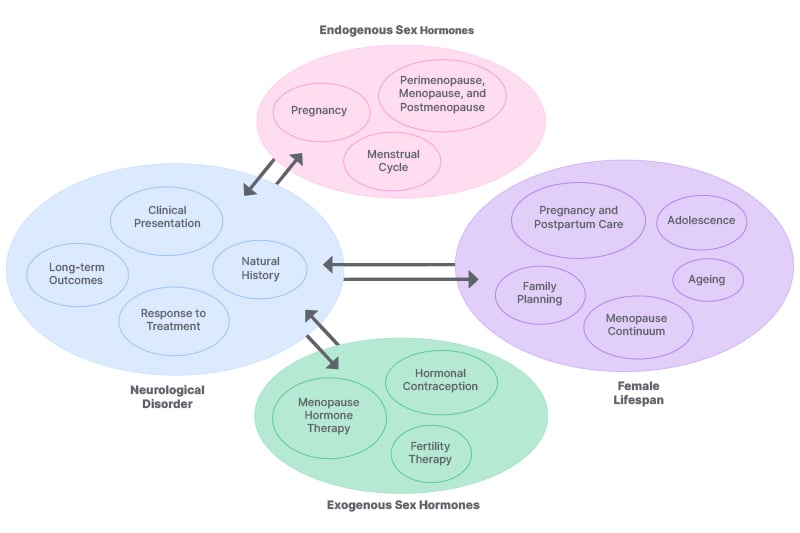
Figure 1: General considerations for female patients.
Significant gaps due to sex bias in research remain. In response to these historical biases, international policies have evolved to mandate the inclusion of sex-based considerations in research. For example, under the Horizon 2020 research framework, the European Commission emphasised the systematic integration of the sex and gender dimension in research and innovation. This directive has been further reinforced by Horizon Europe (2021–2027), which requires grant applicants to include sex and gender considerations in research design, methodologies, and analysis, with the goal of improving scientific quality and societal relevance.17 These policy shifts reflect a growing consensus that sex-disaggregated data and gender-sensitive methodologies are essential to robust, equitable, and precision-based science.
In this review, experts in their respective fields discuss five major groups of neurological disorders to highlight key gaps in neurological care and opportunities to prioritise and advance precision medicine informed by sex-based considerations, as summarised in Table 1.
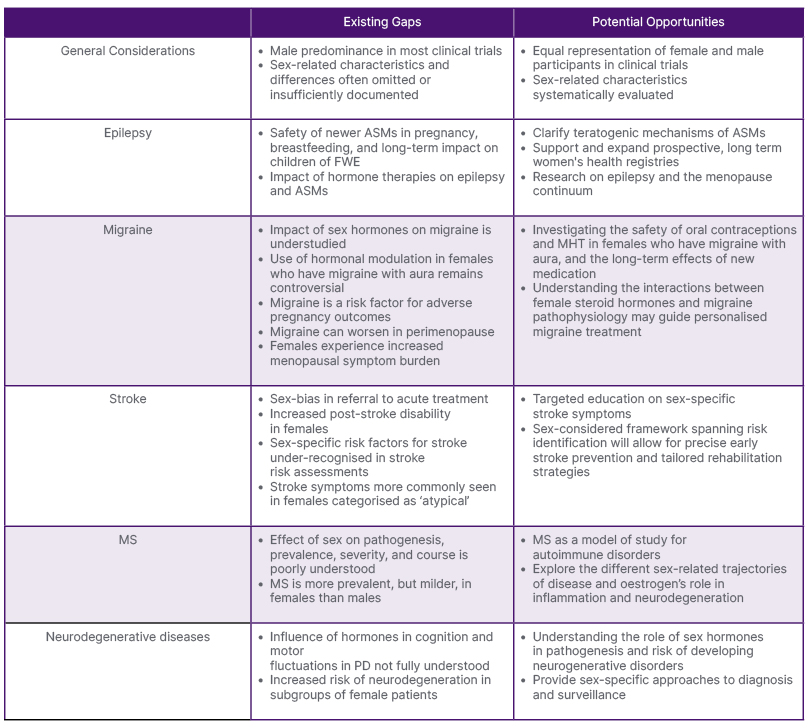
Table 1: Summary of key gaps and opportunities in neurology.
ASM: anti-seizure medication; FWE: females with epilepsy; MHT: menopause hormone therapy; MS: multiple sclerosis; PD: Parkinson’s disease.
These five groups (epilepsy, migraine, stroke, MS, and neurodegenerative diseases) were selected based on their high prevalence and the critical influence of sex-related factors on their pathophysiology, clinical presentation, and management, and are highlighted in Figure 2.
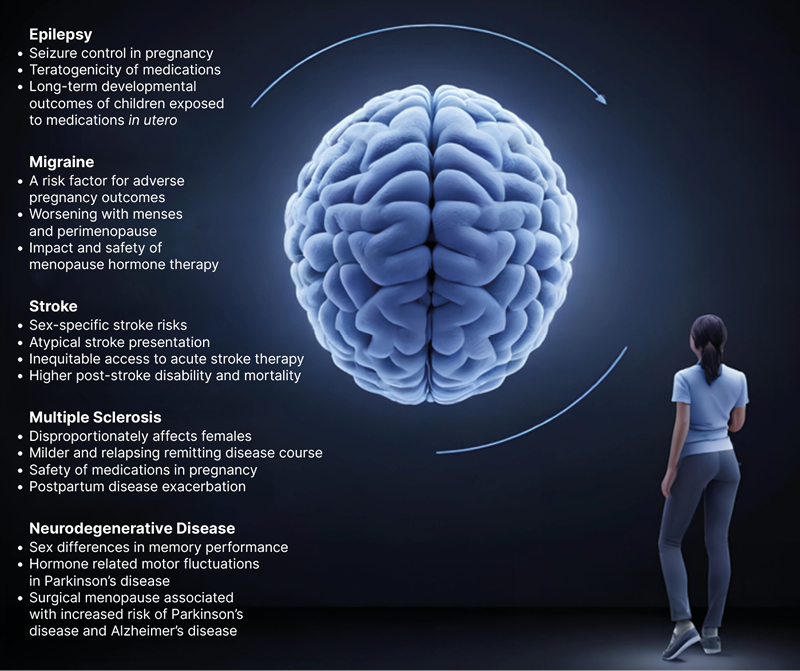
Figure 2: Precision-based medicine in neurology for female patients.
EPILEPSY
Existing Gaps
Of the 70 million people with epilepsy worldwide, at least 15 million are females of childbearing potential (FOCB). Assuming, from epidemiological estimates, that 0.5% of births are to females with epilepsy (FWE), about 650,000 children are born to this population.18 Pregnancy represents multiple, often competing priorities between maternal and fetal health. As seizure control is critical during pregnancy, anti-seizure medications (ASM) should in most circumstances be continued, despite increased risk of major congenital malformations (MCM) and neurodevelopmental disorders.19,20 For example, the unadjusted prevalence of MCMs among lower risk ASMs such as lamotrigine, levetiracetam, and oxcarbazepine ranges from 3.1–3.5%, and can reach as high as 9.7% with valproic acid exposure. This is elevated compared to the 2.4–2.9% rate seen among children of people without epilepsy.21,22 While research on epilepsy and pregnancy has progressed, key gaps remain. Pregnancy data on many newer ASMs remain limited or inconclusive.20-25 For all ASMs, there continues to be a much-needed ability to understand the genetic and epigenetic mechanisms behind their teratogenic potential and impact on long-term neurodevelopment, and to identify subtle cognitive or behavioural effects that may emerge later in life.25-30 Due to restrictions on topiramate and valproate use in FOCB, stemming from their well-documented risks during pregnancy,20,24,25,30,31-39 more data are needed to identify safer and more effective alternatives (including dose-related risks and polytherapy options), as well as the subset of patients who are at the greatest risk for medically refractory seizures, especially with non-valproate treatments.40-44
Regarding prenatal care, at least 0.4 mg of folic acid supplementation remains recommended to mitigate ASM-related MCMs and neurodevelopmental risks, yet the optimal and safe folic acid dosage remains unclear.23,45-48 In postpartum care, while breastfeeding is generally encouraged with emerging lactation safety data for older ASMs, these safety data are still scarce for newer ASMs.18,23
As newer ASMs are increasingly used, awareness of interactions with sex steroid hormones is needed, not only to ensure the safety and effectiveness of exogenous sex steroid hormones from contraceptives, menopause replacement therapy, or assisted reproduction therapies, but also to ensure adequate seizure control in pregnancy. Furthermore, hormonal therapies for catamenial (associated with menstruation) epilepsy and seizure threshold changes during menopause are underexplored.49
Issues beyond reproduction are even more overlooked. Clinical trials rarely analyse sex-specific efficacy and safety of ASMs, as well as both endogenous and exogenous sex steroid hormonal influences in the menopause continuum, resulting in persistent suboptimal care for females across the lifespan. To date, there remains limited evidence to guide the management of females in perimenopause and menopause.50,51
Opportunities for Advancement
To optimise pregnancy outcomes in FOCB, research must prioritise global participation in large-scale ASM pregnancy registries. These should include neurodevelopmental follow-up of offspring through infancy, childhood, and adolescence. Expanding population-based registries beyond Northern Europe, where current population data mostly come from,20,45,46,52-55 is also essential to capture more representative data. To this extent, even the early identification of potential teratogenic risks from newer ASMs through case reports and small case series is crucial.
Preclinical and pharmacogenomic studies, as well as investigations into environmental influences, may clarify teratogenic mechanisms and consequently promote personalised therapy for girls and FOCB. Further clinical studies are needed to expand the identification predictors of response to non-valproate treatments, particularly in genetic generalised epilepsies, to improve individualised treatment strategies and accurately assess risk–benefit profiles.
Bioethical research involving clinicians, ethicists, and patients is critical to support truly personalised counselling. Such frameworks must consider not only biological risk, but also the individual’s values, context, and societal factors. Ethical tools should also guide decision-making in clinical and policy settings.
Additional priorities include multicentre studies on ASM excretion in breast milk, neonatal blood concentrations, and long-term developmental effects. Pharmacokinetic studies must assess interactions between any new ASM and oestrogens and progestins. Multicentric prospective studies are also needed on characterising catamenial epilepsy, including trials on progestin therapy, and on seizure patterns and ASM metabolism during and after menopause. Future ASM trials must systematically assess sex-based differences in drug response and adverse effects.
MIGRAINE
Existing Gaps
Migraine is predominantly a disorder impacting females, although males with migraine are often overlooked and underrepresented in clinical trials.56 According to the Global Burden of Disease (GBD) database study, migraine is the leading cause of disability-adjusted life years among FOCB,57 with nearly one in every four individuals experiencing migraine. Studies have shown that migraine attacks occurring perimenstrually tend to be more disabling, longer lasting, associated with more bothersome symptoms, and are more refractory to treatment compared with migraine at other times of the menstrual cycle.58
During pregnancy, migraine is a risk factor for adverse pregnancy outcomes. In a prospective study of 10,038 females who were nulliparous, a self-reported history of migraine was associated with 26% higher odds of adverse pregnancy outcomes. This effect is mediated predominantly by hypertensive disorders of pregnancy such as pre-eclampsia and preterm birth.59 Currently, according to the United States Preventive Services Task Force (USPSTF), migraine is not considered a risk factor that would prompt the consideration of low-dose aspirin for pre-eclampsia prevention.60
Following reproductive years, during the menopausal transition, migraine tends to worsen. In addition to experiencing more frequent and severe migraine attacks during perimenopause, females with migraine also experience increased vasomotor symptoms, mood disturbances, and sleep disruption.61
Despite the distinct influence of hormonal epochs on migraine and the impact of migraine on the risk of vascular events, treatment options for migraine are not specific to the dynamic physiologic changes exacerbating migraine. These milestones are often neglected in clinical practice. For FOCB, clinicians do not routinely inquire about reproductive plans or contraceptive use, leaving patients at risk for conceiving while on potentially teratogenic medications. While pregnant, patients experiencing migraine are often left without appropriate and safe migraine treatment options, in part, reflecting the paucity of robust clinical studies on the safety of migraine treatments in pregnancy. Perimenopausal symptoms, which significantly contribute to migraine, are often overlooked by clinicians. In addition, the risk of medications, in particular calcitonin gene-related peptide antagonists, to bone health and vascular health has not yet been well explored.62,63 This class of medications may adversely impact both organ systems, which are more vulnerable within the menopause continuum. Furthermore, data on sex-related differences in efficacy have been raised, with these drugs being less effective in the acute treatment of male patients.64
Opportunities for Advancement
Further understanding the influence of hormones on migraine pathophysiology represents a critical opportunity towards precision-based migraine treatment. The potential impact on migraine of various forms of hormonal manipulation (through hormonal contraception or hormone therapy) in the menopause continuum is understudied and deserves attention to help identify formulations with more favourable outcomes for migraine and more favourable safety profiles. To date, multiple societies, including the European Headache Federation (EHF) and the WHO, recommend against the use of combined hormonal contraceptives for females who have migraine with aura.65 Yet, the International Headache Society (IHS) suggests that low dose oestrogen can be considered for females who have migraine with visual aura on a case-by-case basis.66,67 With many females either taking or desiring hormonal contraception with oestrogen, and the renewed interest in menopause hormone therapy, a more in-depth appreciation for sex-specific risk of stroke at distinct doses and formulations of oestrogen represents a critical opportunity to better inform safe and evidence-based care of females with migraine.
STROKE
Existing Gaps
Despite advances in stroke research, precision medicine remains inadequately tailored to females, resulting in significant disparities in prevention, diagnosis, treatment, and outcomes. Similar to cardiac disease, females often present with stroke symptoms that are considered atypical, such as fatigue or mental status changes, leading to misdiagnosis and delayed treatment.16 Consequently, they are less likely to receive timely interventions like thrombolysis, or be admitted in high-intensity care units, despite evidence suggesting that they may benefit more from such treatments.16,68,69
The underrepresentation of females in clinical trials further exacerbates these issues. Studies reveal that females are enrolled at lower rates than male counterparts, particularly in trials focusing on stroke treatment, intracerebral haemorrhage, and rehabilitation.69 This lack of representation limits the applicability of research findings to female patients and hinders the development of sex-specific treatment protocols.
Moreover, sex-specific risk factors, such as pregnancy-related complications, hormonal therapies, and migraine, are often under-recognised in stroke risk assessments and inadequately targeted with prevention strategies.70 Additionally, females are more likely to experience worse recovery post-stroke, influenced by factors like older age at onset, higher prevalence of comorbidities, and social determinants of health.68,70
Recovery outcomes also differ by sex. Females are generally older at the time of stroke onset, with an average age of 72.9 years compared to 68.6 years for males. This age difference contributes to higher post-stroke disability and mortality rates among females.16,68,71-73 For instance, within 3–6 months post-stroke, 15% of females are unable to eat independently compared to 9% of males, 37% of females require assistance with dressing versus 20% of males, and 32% of females need help transferring from bed to chair compared to 13% of males.16,68,71-73 These disparities underscore the need for sex-specific approaches in stroke prevention, treatment, and rehabilitation to improve outcomes.
Opportunities for Advancement
Despite progress in stroke care, significant opportunities remain to enhance outcomes for females across the continuum of cardiovascular prevention, hyperacute treatment, and rehabilitation. A precision medicine approach must acknowledge sex-specific risk factors, including lower access rates to rapid diagnostic pathways, lower socioeconomic status,74 higher prevalence of atrial fibrillation as a stroke aetiology in female patients, adverse pregnancy outcomes, and hormonal transitions like menopause, all of which disproportionately affect stroke risk in females.
In the realm of primary and secondary prevention, sex disparities in medication adherence are critical. Chen et al.75 reported that females had significantly higher nonadherence to cholesterol-lowering (prevalence ratio: 1.80; 95% CI: 1.14–2.84) and antiplatelet therapies (prevalence ratio: 1.53; 95% CI: 1.003–2.34) at 90 days post-stroke.75 This gap may stem from differences in health beliefs, caregiver responsibilities, and socioeconomic barriers. Tailored interventions, including patient education and culturally competent care models, are vital to improve adherence and reduce recurrent stroke risk.
Hyperacute stroke management presents another opportunity. Although females tend to present with more severe strokes, when matched for baseline risk factors, the outcomes after endovascular thrombectomy do not significantly differ by sex (odds ratio for good functional outcome: 0.89; 95% CI: 0.66–1.2; symptomatic intracranial haemorrhage odds ratio: 1.00; 95% CI: 0.44–2.26).76 Nevertheless, females are still less likely to receive endovascular thrombectomy,69,77 and delays due to atypical symptom presentation and lower prehospital recognition contribute to worse outcomes.78 Targeted education for both clinicians and the public on sex-specific stroke symptoms could help bridge this gap.
Rehabilitation strategies must also evolve. Females experience higher rates of post-stroke disability, depression, and reduced access to outpatient therapy.68 Integrating mental health services and caregiver support into rehabilitation planning could mitigate these disparities. Furthermore, rehabilitation trials must increase female enrolment and disaggregate data by sex to inform best practices.78
Optimising stroke care for females requires a sex-considered framework spanning risk identification, equitable acute intervention, and individualised recovery plans. Institutional policies and clinical research must reflect these imperatives to close the persistent outcome gap.
MULTIPLE SCLEROSIS
Existing gaps
Multiple sclerosis (MS) disproportionately affects females, with a female to male ratio of 2–3:1. This sex disparity has been rising in recent years, but the precise factors driving this increasing incidence remain unclear. Understanding these factors is crucial, as both genetic and environmental influences likely contribute to this trend.79
Although it is well-established that MS progresses differently between males and females, the influence of sex hormones on prognosis is underexplored.80 A recent large-scale survey on women’s health issues for individuals with MS identified menopause, pregnancy, and hormonal treatments as priority research areas, underscoring the need for further exploration into how these factors affect MS progression.81
While the impact of MS on ovarian reserve remains poorly understood, significant research is emerging on pregnancy and breastfeeding. However, much remains unknown regarding the safety of disease-modifying therapies, highlighting a need for treatments that can offer protective effects during these stages of a female’s life.82 Further research is also required to investigate how menopause interacts with MS, particularly in terms of symptom management and disability worsening.83,84
Finally, regional differences in grey matter atrophy suggest sex-specific mechanisms in disease progression, emphasising the need for more targeted research on brain volume dynamics in MS.85,86 Understanding these sex differences in brain atrophy is crucial, and linking this research with other areas of neurology could provide broader insights into neurodegenerative diseases.
Opportunities for Advancement
MS is more prevalent in females, but more disabling in males. Moreover, while females are more commonly affected by a relapsing-remitting course, males tend to experience progressive forms more frequently. Exploring the biological mechanisms behind these sex differences presents a significant opportunity to uncover disease pathogenesis.87,88 Indeed, females living with MS might pose as a model for understanding neurological autoimmune diseases, as MS onset typically occurs in young females and persists throughout their entire lifespan. This may also present a unique opportunity to understand disease progression in the context of hormonal fluctuations across different life stages, from adolescence to menopause.89
The difference in disease progression across sexes in MS warrants further investigation into mechanisms like the glymphatic system, which appears to be impaired in progressive forms. This system could be crucial in understanding neurodegeneration in females with MS, and may offer a new therapeutic target for disease progression.90
Exploring neuroprotective treatments, particularly those that leverage oestrogen’s known effects on neuroprotection and remyelination, presents an exciting avenue for research. Trials exploring oestriol’s effects on cognitive function and its combination with disease-modifying therapies have demonstrated promise, though further studies are needed to confirm long-term benefits.91,92 Similarly, the investigation of selective oestrogen receptor modulators offers hope for treatments that can protect and repair the myelin sheath in females with MS.93
Together, these opportunities highlight the need for continued exploration into sex-specific therapeutic strategies, considering hormonal influences and females’ unique biology. With a deeper understanding of these mechanisms, treatment outcomes for females with MS can be improved, ultimately advancing precision neurology.
NEURODEGENERATION
Existing Gaps
AD and other neurodegenerative diseases (Parkinson’s disease [PD] being the fastest growing one) are the largest contributors to neurological health loss.94 In both AD and PD, females have traditionally been underrepresented in clinical trials.95,96 However, although concerning knowledge gaps persist, the evidence on sex-related differences in the pathophysiology, natural history, and management of neurodegenerative diseases is emerging.97
For example, in a recent meta-analysis of six studies with a total of 1,376 participants with AD (55% female), female sex was associated with faster tau accumulation localised to inferior temporal (β=−0.14; 95% CI: −0.22–-0.06; p=0.009), temporal fusiform (β=−0.13; 95%CI: −0.23–-0.04; p=0.02), and lateral occipital regions (β=−0.15; 95%CI: −0.24–-0.06; p=0.009) compared with male sex, possibly suggesting a heightened risk of cognitive decline in females compared to males.98 At the same time, healthy females outperform males in neurocognitive tests. Sex differences in memory performance, particularly verbal, begin in childhood, and grow larger in effect size just post-puberty.99 Although these differences decrease with menopause, a certain degree of female advantage remains in the healthy ageing brain through midlife and old age, possibly leading to the underdiagnosis of AD-related mild cognitive impairment and dementia among females.98,100
Substantial sex-based differences also exist in PD. While the incidence of PD is, in general, higher in males compared to females, it increases significantly in females who underwent hysterectomy and bilateral oophorectomy before menopause.101 Females with PD are at increased risk of developing highly disabling motor complications, including motor fluctuations, dyskinesias, and non-motor fluctuations (especially depression/anxiety, sleep/fatigue, and dysautonomia domains), compared to male patients. However, females remain disproportionally underrepresented in referrals for device-aided therapies compared to the general PD population.102-105 Furthermore, although catamenial fluctuations in PD are a phenomenon that has been described since the 1980s,106,107 little is known regarding the impact of both exogenous and endogenous hormones in disease management and progression. On the other hand, male patients with PD have a higher risk of cognitive impairment in comparison to female patients.108
Although PD is predominantly affecting elderly individuals, it can also, particularly in its monogenic forms, affect FOCB. Data regarding the safety of drugs used in the management of PD in pregnancy are still scarce,109 and multicentred registries are lacking.110
Opportunities for Advancement
As data emerges to implicate the role of neuroactive sex steroid hormones, such as 17β-oestradiol, and their endogenous fluctuations in learning and memory, clinicians need to account for these differences in neuropsychological testing when assessing cognitive impairment.100 Consequently, sex-specific timing considerations need to be included into indications for anti-Aβ and anti-tau treatments in a more personalised approach for females with AD.98
In PD, α-synuclein seed amplification assays are a novel tool enabling the biochemical diagnosis of the disease. They also allow for detecting individuals who are already at prodromal stages of PD.111 Females who underwent hysterectomy and bilateral oophorectomy before menopause, and are thus at heightened risk of developing AD and PD alike, should be followed up, assessed with seed amplification assays early, and be included in RCTs of disease-modifying molecules. Concerning treatment for symptomatic PD, there are no available guidelines for the treatment of pregnant patients with PD or specifically addressing the higher risk to develop dyskinesia and specific impulse compulsive behaviour. Sex should be considered when selecting device-aided therapies for people with PD.104 Importantly, the reasons for inappropriately less frequent referrals of females for device-aided therapies need to be unearthed and strategically tackled. Moreover, non-motor symptoms are differently affecting male and female patients with PD.109
MAPPING A WAY FORWARD: PRECISION-MEDICINE FOR HALF THE POPULATION
Specifically addressing existing gaps and opportunities by integrating sex-based considerations in neurology across clinical care, research, and neurology training will ultimately advance precision medicine for all.112 While this review has focused on sex-specific or biological considerations in females affected by neurological conditions, gender considerations are rarely isolated from sex considerations.
As such, the authors’ review does not explore the interconnectivity of sociodemographic factors influencing sex-considered health measures. In fact, gendered aspects such as a woman’s employment and education status, as well as her marital and caregiver status, can contribute to observed gender disparities in clinical trial participation, which prompts a call for gender-informed study recruitment methods.113 Gender-specific considerations, such as disproportionate caregiving, paid work constraints, and cultural norms, are likely to play a part in women’s ability and willingness to enrol in clinical trials. Although out of the scope of this review, gendered aspects of bias should also be addressed to increase parity on research, such as flexible or remote visit options and stipends for childcare, transportation, or lost wages.114
Furthermore, important male-specific patterns (such as higher rates of cluster headache and higher risk of primary progressive MS in male compared to female patients) are acknowledged but remain outside the scope of this review.
Clinical Care
In clinical care, there is an urgent need to develop neurology clinical practice guidelines that incorporate female-specific health issues across the lifespan. It is notable that, while sex-specific considerations extend beyond reproduction, reproductive health-related research constitutes the majority of existing sex-considered neurological research.10 Female-specific considerations must be integrated into diagnostic criteria, as numerous neurological disorders present differently in females compared to males, leading to risks of misdiagnoses and inappropriate treatment. In females with neurological disorders, the interactions between neuroactive sex steroid hormones and their endogenous fluctuations throughout reproductive life and the menopause continuum need to be emphasised. Precision-based medicine for females with neurological disorders requires sex-specific data and guidelines during different hormonal life stages. Applying a comprehensive precision medicine approach, neurological history taking needs to consider symptoms across the menstrual cycle and discuss potential interactions between oral contraceptives or menopause hormone therapy and neurological symptoms. Knowledge on the safety of medications during family planning, pregnancy, and breastfeeding is essential. This includes addressing the teratogenic risks, the potential impact on fertility, and maternal and fetal safety. In cases where medications are contraindicated in pregnancy or during hormonal transitions, neurologists must be familiar with safe and effective alternatives, including non-pharmacological strategies. Females with neurological disorders must be included in shared decision-making together with their neurologists on reproductive choices, contraceptives, or menopause hormone therapy use during different hormonal life stages. Input from endocrinology, obstetrics/gynaecology, and psychiatry in multidisciplinary care models can help neurologists navigate the complex overlay between neurological symptoms and hormonal health.
Research
In research, to ensure findings are sex-specific, fixed quotas (for example, in accordance with the epidemiological representation of sexes) for the inclusion of females in clinical trials are urgently needed across all areas of neurology. This could be achieved through funding agencies and regulatory bodies requiring sex-considered research designs and incentivising studies that investigate neurological conditions, specifically in females. Furthermore, sex-disaggregated data need to be analysed in clinical trials, and hormonal status (e.g., premenopausal, perimenopausal, postmenopausal, hormonal therapy use, and pregnancy) should be systematically documented and incorporated into trial design and interpretation. Research must systematically consider the impact and potential interactions of hormonal changes across the lifespan on neurological symptoms, as well as on the effectiveness and tolerability of neurological medications. Furthermore, prospective registries are urgently needed to document the safety of neurological medications in the context of family planning, pregnancy, and breastfeeding. Technology should be leveraged to advance sex-specific research; for instance, AI could be used to reanalyse older datasets for sex-based differences, while electronic medical records offer new opportunities to systematically integrate sex considerations into clinical research design.
Neurology Training
In education, there is the opportunity to disseminate clinical guidelines and research to health practitioners, as well as train the next generation of experts informed on sex- and gender-specific considerations. The European Academy of Neurology (EAN)’s Diversity, Equity and Inclusion coordinating panel reviewed all the education programmes in Europe on sex and gender education and identified a scarcity of sex and gender specific topics in medical curricula.17 From undergraduate medical training to postgraduate neurology training and continuing medical education, topics related to sex-specific influences on neurological presentation, disease trajectory, response to therapy, and long-term outcomes must be systematically integrated alongside multidisciplinary efforts with endocrinology, obstetrics, gynaecology, and psychiatry. Medical education must emphasise the importance of sex-specific diagnostic criteria and the impact of sex steroid hormones on neurological conditions. In the USA and Canada, a dedicated Women’s Neurology curriculum has been developed and is endorsed by the American Academy of Neurology (AAN), marking an important step towards formalising sex- and gender-based neurological education. Similar initiatives beyond the North Americas are urgently needed.
In advancing sex-specific considerations, there is an exciting chance to open new pathways for a more precise understanding of neurological illness. Neurology has an important opportunity to prioritise a more expansive and inclusive understanding of sex-specific differences, and in doing so, everyone stands to gain from implementation of precision medicine at a fundamental level.







