Abstract
While hormonal changes have been recognised to influence seizure control and have been studied in association with menstrual cycles and pregnancy, there is a paucity of data on the menopause transition in epilepsy. Given the known effects of sex steroid hormones on neuronal excitability, their endogenous fluctuations during perimenopause, as well as menopause hormone treatments, may alter seizure control. Epilepsy may also be associated with premature ovarian insufficiency and early menopause. This is especially important for epilepsy-related comorbidities, for which menopause can constitute a second hit, such as osteoporosis. Additional considerations for females with epilepsy across the menopause continuum include changes in antiseizure medication clearance and potential interactions with menopausal hormone therapy or other concomitant medications. This comprehensive review summarises the currently available literature on epilepsy and menopause, highlights gaps in knowledge, and underscores the need for research efforts, particularly longitudinal studies investigating the menopause transition.
Key Points
1. Given the known influence that endogenous sex hormone fluctuations and exogenous hormone therapies can have on seizure susceptibility and antiseizure medication clearance, there are many important clinical considerations regarding the menopause transition for females with epilepsy.2. The article summarises the currently available literature on epilepsy and menopause, including the influence of epilepsy on the timing of menopause and associated comorbidities; considerations about how the menopause transition may influence seizure frequency and antiseizure medication clearance; and the use of menopausal hormone therapy.
3. The relationship between menopause and epilepsy is complex. The currently available literature is sparse, and there is a lack of evidence-based practice guidelines for managing epilepsy through the menopause transition. Further studies, including longitudinal studies that follow females with epilepsy from their reproductive years through the menopausal continuum, are necessary to better understand the relationship between epilepsy and menopause.
INTRODUCTION
Epilepsy is a serious and common neurological condition, characterised by recurring, unprovoked seizures, affecting approximately 25 million females worldwide.1 Historically, the term ‘women’ has been used to encompass both sex- and gender-specific issues in epilepsy. In this paper, the term ‘female’ will be used where appropriate to highlight sex-specific considerations in epilepsy. Sex-specific research studies of people with epilepsy have focused primarily on care during the reproductive years, while few studies have addressed hormonal influences beyond reproductive stages, such as perimenopause, menopause, and post-menopause, collectively known as the menopause continuum. Sex steroid hormones (SSH) exert unequivocal effects on neuronal excitability. Oestrogen increases excitation through its effects on glutamate receptors. In contrast, progesterone neuroactive metabolites, such as allopregnanolone, are positive modulators of γ-aminobutyric acid (GABA) receptors, thereby potentiating inhibition. However, activation of progesterone receptors may increase excitation through effects on α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, reflecting the complex molecular actions of progesterone.2 The ability to identify individuals with heightened sensitivity to hormonal fluctuations and personalise treatment is limited. These challenges are especially pronounced during perimenopause and menopause.3
Fluctuations in SSH, such as oestrogen and progesterone, during perimenopause and menopause may affect both neuronal excitability and antiseizure medication (ASM) clearance, which can additionally impact the frequency and severity of seizures. This is further influenced by individual variability in hormonal fluctuations, sensitivity to SSH changes, and age-related alterations in ASM metabolism and clearance.
This article will review epilepsy in the context of SSH changes during perimenopause and menopause; the complex relationship between ASMs, SSHs, and bone health; and key gaps and opportunities for future research to personalise care for individuals during perimenopause and menopause.
METHODS
An extensive search was done using the search terms ‘epilepsy’ and ‘menopause’ to gather articles from 2006–2023 on PubMed, Ovid, and the Cochrane Library for analysis. Key articles published prior to 2006 that pertained to this subject were also included within the scope of this approach. The authors selected predominantly original research articles with relevant information on the metabolic pathways of ASMs and pharmacokinetic changes in pregnancy/postpartum, with occasional relevant reviews included. The initial selection was further supplemented with studies identified by co-authors to be relevant for inclusion.
The article was reviewed and edited by expert clinicians and scientists from the Epilepsy in the Childbearing Age Through Menopause (ECAM) Consortium – Menopause Committee. ECAM is an international consortium of clinicians and scientists with the mission to advance optimal care for patients with gestational potential and epilepsy across their lifespan, by fostering interdisciplinary research and clinical collaborations across participating institutions.
SEX STEROID HORMONES AND EPILEPSY
Females with epilepsy present unique challenges due to the cyclical fluctuation of SSH concentrations and their metabolites throughout the lifespan. Such hormone shifts can impact seizure susceptibility in a complex manner, as highlighted in the authors’ recent article on the biosynthesis, regulation, and mechanisms of action of such hormones and their impact on epilepsy.4
Sex Steroid Hormones During Transition to Menopause
The pattern of SSH fluctuations is divided into three biological stages: (1) premenstrual/pre-menarche years; (2) menstrual/reproductive years; and (3) menopausal years, with two main transition periods: adolescence and perimenopause. Perimenopause is a key transitional period involving increased anovulatory cycles with fewer cyclic progesterone elevations and unpredictable oestrogen surges that can last up to 10 years.
Although SSHs fluctuate during the perimenopause years, the extent and pattern of these fluctuations remain poorly understood. In 2001, a multidisciplinary group of experts convened a Stages of Reproductive Aging Workshop (STRAW), establishing the STRAW criteria, a consensus-derived staging system that defines reproductive, perimenopausal, and menopausal transition, as well as postmenopausal stages, and which has been updated in subsequent years.5 The STRAW criteria were derived from cohort studies of midlife females with chronic illness and endocrine disorders, examining changes in menstrual, endocrine, and ovarian markers. These markers included anti-Müllerian hormone, inhibin-B, follicle-stimulating hormone, and antral follicle count, and they established stages for the menopausal transition and postmenopausal phase. The STRAW criteria enable a standardised terminology for these important life stages. The authors’ review will highlight epilepsy in the context of these reproductive life stages, as summarised in Figure 1.
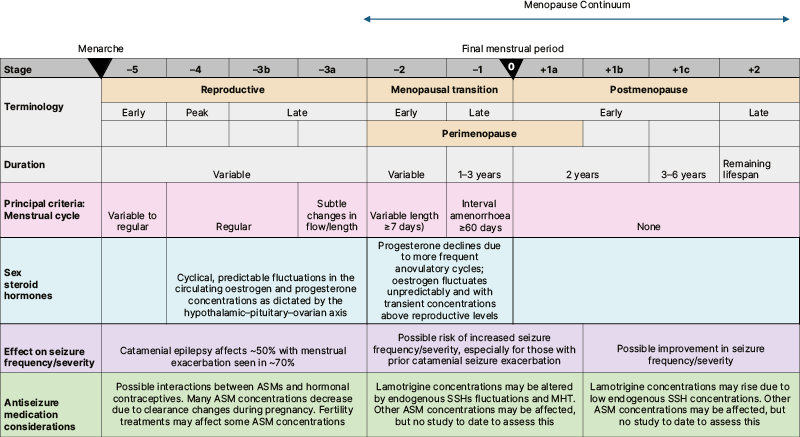
Figure 1: Stages of reproductive ageing and clinical considerations for females with epilepsy.
Adapted from Harlow SD et al.4 used under CC BY-NC 4.0.
ASM: antiseizure medication; MHT: menopause hormone therapy; SSH: sex steroid hormone.
The Menopause Transition
The effect of epilepsy on the timing of reproductive cycling cessation
Epilepsy may affect reproductive health, and while data on fertility is equivocal,6,7 several lines of evidence support a higher rate of premature ovarian insufficiency and early menopause in females with epilepsy. In one study, females with epilepsy (N=50; 41 with focal and nine with generalised epilepsy), interviewed about symptoms and assessed with endocrine markers, had a significantly higher rate of non-surgical premature perimenopause and menopause compared with control females without epilepsy (14% versus 3.7%; p<0.042). The mean age of estimated premature ovarian insufficiency in the epilepsy cohort was 39.6 years (range: 37–42 years), with no clear correlation with epilepsy duration, severity, or ASM type. Interestingly, the only clinical factor that correlated with premature ovarian insufficiency was a history of catamenial seizure exacerbation (pattern aligned with the cyclic hormonal fluctuation during the menstrual–ovarian cycle; p<0.02).8
Yet another study, which surveyed a cohort of females with epilepsy aged ≥45 years, raised concern that a higher seizure burden was associated with the earlier cessation of reproductive cycling (p=0.014). No correlation with ASMs was observed, consistent with the previous study. This study concluded that seizures may disrupt hypothalamic and pituitary function or alter neurally mediated trophic effects on the ovary.9
The course of epilepsy during perimenopause and menopause
Assuming a simplified view that progesterone, through the metabolite allopregnanolone, is predominantly an anticonvulsant, while oestrogen, through the metabolites oestradiol and oestrone, is a proconvulsant, the expected outcome is that perimenopausal females experience unpredictable periods of heightened seizure intensity and frequency.4 It is conceivable that seizure worsening is more likely to happen in early perimenopause, with higher amplitude oestrogen surges, and less so in late perimenopause, when the concentrations settle towards lower values. Indeed, females with catamenial epilepsy, thought to be more sensitive to hormonal fluctuations, were shown to have a higher risk of seizure worsening during perimenopause, followed by an improvement in seizure frequency and severity at the completion of menopause.9-11
Around half of females with epilepsy have a catamenial pattern,12,13 while about two-thirds of all females have seizure worsening during perimenopause.10 A cross-sectional study using mailed questionnaires completed by 42 respondents evaluated the course of epilepsy from reproductive years through perimenopause and menopause, and concluded that the high percentage of females in the perimenopausal group who took synthetic hormone replacement therapy were significantly associated with an increase in seizures (p=0.001).10 This group went on to investigate the effect of menopausal hormone therapy on seizures at this stage in life.14
In contrast, a cross-sectional study employing a structured interview of 61 participants in the menopause continuum (46 postmenopausal and 15 perimenopausal)15 found mixed results regarding seizure frequency at menopause. Among the 49 participants with epilepsy onset before menopause, 40.8% reported seizure worsening, 26.5% reported improvement, and 32.6% reported no definitive change at menopause. Interestingly, 12 (20%) of the 61 females from the study reported that seizures first began during or after menopause, with eight having no proven cause for their epilepsy.
Additional SSHs of importance through the menopause continuum, though less commonly considered, are testosterone and its metabolites, androstanediol and oestradiol.16 During menopause, testosterone serum concentration changes as well, affecting sexual function, mood, bone health,17 and possibly neuronal excitability and seizure control.
With the limited available data, females with epilepsy may have an increased risk for seizure worsening at perimenopause and possibly a lower risk in the postmenopausal period. In the absence of definitive staging of the menopause continuum and therapeutic strategies to address this problem, there are currently no guidelines available for creating a personalised treatment plan during this vulnerable period.
Menopausal Hormonal Therapy
Current MHT employs combined oestrogen-progestin therapy for people with an intact uterus (who need a progestin to prevent oestrogen-related endometrial hyperplasia) and oestrogens alone for females who have undergone hysterectomy.18 The use of MHT at menopause decreased significantly after the initial findings of the Women’s Health Initiative (WHI) published in 2002,19 which reported an increased risk for invasive breast cancer. After conducting an evidence-based analysis of the subsequent literature in the following two decades, the 2022 North American Menopause Society position statement acknowledged that the risks of hormone therapy differ depending on type, dose, duration of use, route of administration, timing of initiation, and whether a progestin is used.20 Their consensus recommendations emphasise the importance of MHT as the most effective treatment for vasomotor and genitourinary symptoms, and it has been shown to prevent bone loss and fracture. For females aged younger than 60 years or those within 10 years of menopause onset, with no contraindications, the benefit–risk ratio is favourable for the treatment of menopause-related signs and symptoms, as well as prevention of bone loss.
The effect of menopausal hormone therapy in people with epilepsy
Given evidence that people with epilepsy may be at risk of early menopausal onset8,9 and have multifactorial risks for osteoporosis,22 consideration of MHT is important. An additional concern for people with epilepsy is the influence of exogenous SSH administration on seizure control. One randomised, placebo-controlled, double-blind clinical trial investigated the effect of conjugated equine oestrogens/medroxyprogesterone acetate (0.625 mg of conjugated equine oestrogens (CEE/MPA) plus 2.5 mg of medroxyprogesterone acetate) compared with placebo on seizure frequency in postmenopausal females with epilepsy.22 The study was halted before the enrolment target, after publication of the WHI study results in July 2002.19 Despite the low numbers (n=21), the study demonstrated a significant conjugated equine oestrogens/medroxyprogesterone acetate dose-related increase in seizure frequency in postmenopausal females with epilepsy, as well as altered ASM levels in two patients.
Another interview-based cross-sectional study yielded conflicting findings: 31/61 (51%) of menopausal patients had used MHT (MHT-specific details were not provided and likely various preparations were used), 22 (71%) experienced no changes in seizure frequency, five (16%) showed improvement, and four (13%) reported worsening.15
A recent systematic review summarised what is known about the impact of MHT on seizure frequency, noting only three human studies and limited evidence available.22 Despite the limited evidence, at perimenopause, some experts may use forms of MHT to suppress hormonal fluctuations and help with seizure control when other therapies fail. This approach has a biological rationale due to the shared biology with catamenial epilepsy. As more MHT options emerge, with guidelines supporting their use in the general population, there is a pressing need for more research due to the unique implications of MHT on seizure control.
SEX STEROID HORMONES AND ANTI-SEIZURE MEDICATIONS
ASMs provide the foundation for the medical treatment of epilepsy. When selecting an ASM, there are multiple primary factors that are considered, including the type of epilepsy (generalised or focal), childbearing potential, comorbidities, and concurrent medications. Current practice often overlooks the impact of changes in SSHs, which could have a significant effect on medication metabolism and possibly efficacy, depending on the patient’s biological stage.
Pharmacokinetic Changes During Transition to Menopause
There is a bidirectional interaction between ASM and SSHs. ASMs that induce hepatic enzymes (such as cytochrome P450 3A4 [CYP3A4]) and increase sex hormone-binding globulin (SHBG) accelerate SSHs metabolism and lower their circulating concentrations, which may be relevant when considering MHT options. Conversely, there is solid evidence from pregnancy studies that SSHs affect the clearance of many ASMs.23-25
There are several additional broader considerations for older people with epilepsy, such as heightened sensitivity to neurocognitive adverse effects, ASM interactions and reduced metabolism, deleterious effects on bone health, and cholesterol homeostasis.27-28 People with epilepsy reported better quality of life on monotherapy compared to polytherapy.29 It may be beneficial to transition to monotherapy to improve quality of life; however, such changes must be carefully balanced with the need to maintain stable seizure frequencies.
The effect of ageing on glomerular filtration rate (GFR) is well known with a gradual decrease occurring over time, which is not specific to patients with epilepsy. Although glomerular filtration is directly proportional to body weight, there may also be weight-independent sex differences in GFR.30 Importantly, females with an earlier onset of menopause have a higher incidence of decreased GFR than females with a longer reproductive period.31 Therefore, ASMs that are predominantly renally-leared, for example, levetiracetam, lacosamide, and zonisamide, may theoretically accumulate to higher serum concentrations in postmenopausal years when compared to reproductive years.
Lamotrigine and Sex Steroid Hormones
Lamotrigine has a bidirectional interaction with SSHs. Oestrogen can reduce serum lamotrigine concentrations, and lamotrigine can decrease sex hormone concentrations, in particular progesterone,32 though the effect is modest.
Prior work on hormonal contraception and lamotrigine interactions,33,34 as well as pregnancy-related changes in lamotrigine clearance35,36 demonstrated that UGT-glucuronidases, which metabolise lamotrigine, are influenced by oestrogen concentrations. Glucuronidation is highest at the end of pregnancy, and it is likely to be significantly decreased at menopause due to low oestrogen concentrations. Indeed, one study with samples from 507 females and 302 males taking lamotrigine found that clearance as a function of bioavailability increased after age 18 years, peaked at 36 years, and gradually declined with advancing age.37 Another study of dose/concentration ratios for 752 males and 1,115 females on lamotrigine showed a decline in females aged 51–55 years, when dose/concentration ratios became significantly lower among females than among males in this age group (p<0.05).38
The interaction between lamotrigine and oestrogen is especially important when considering the use of MHT at menopause. Exogenous oestrogen may lead to an increased lamotrigine clearance, resulting in low lamotrigine concentrations, which may exacerbate seizures. The previously mentioned CEE/MPA trial in epilepsy found that patients taking lamotrigine in the treatment arm not only had an increase in seizure frequency, but also a decrease in lamotrigine concentrations by 25–30%.14 Findings were reinforced by a retrospective study that analysed 79 users of lamotrigine and MHT, and found that oestradiol/oestriol-based MHTs reduced lamotrigine serum concentration-to-dose ratios.39 The magnitude of this interaction is variable and has been attributed to interindividual differences in the hepatic glucuronidases involved in lamotrigine metabolism.35,40
Despite the above data, lamotrigine serum concentrations are not routinely monitored during perimenopause, when they are likely to fluctuate. Similarly, when patients attain postmenopausal status, if on the same dose, they most likely have higher serum concentrations than during reproductive years and may benefit from a dose reduction to prevent risk of toxicity.
Enzyme-Inducing Anti-Seizure Medications and Sex Steroid Hormones
Strong (e.g., phenobarbital, primidone, phenytoin, carbamazepine, oxcarbazepine, cenobamate) and weaker hepatic enzyme CYP3A4 inducers (e.g., topiramate, perampanel, clobazam) increase the metabolism of SSHs, resulting in lower concentrations. Some ASMs (e.g., carbamazepine, oxcarbazepine) also induce SHBG, which results in reduced SSH concentrations.41 Therefore, regimens including any of these ASMs may be expected to require adjustments in the dosage of hormone therapy.
BONE HEALTH AND OTHER COMORBIDITIES AT MENOPAUSE
Bone Health During the Menopause Continuum
Oestrogen regulates bone remodelling by inhibiting osteoclasts and is essential for bone health.42 Menopause is known to be the most important risk factor for bone loss in females. The lack of ovarian-derived oestrogen after menopause accelerates bone mineral density loss compared to males and significantly increases the risk of fractures. Osteoporosis is defined as a bone mineral density 2.5 standard deviations below gender matched controls (T-score < –2.5), and osteopenia is defined as a T-score between –1 and –2.5. Approximately 30% of postmenopausal females in the USA have osteoporosis, and 54% have osteopenia.43
Epilepsy is associated with a significant increased risk of osteoporosis and has been shown to be associated with a two- to six-times increased risk of fractures.44 The underlying pathophysiology of this increased risk is not completely understood, and multiple mechanisms are likely involved. The increased risk of fracture is present even when excluding seizure-related fractures.44 ASMs have been found to contribute to the increased risk of metabolic bone disease in people with epilepsy.45 Enzyme-inducing ASMs lead to reduced concentrations of vitamin D and can also reduce the concentrations of oestrogen and testosterone by increasing SHBG concentrations, mechanisms that contribute to bone loss.44 Non-enzyme inducing ASMs, such as valproate, have been found to impact bone health as well. In a cross-sectional study of premenopausal females, carbamazepine, phenytoin, and valproate were associated with significantly lower concentrations of calcium compared to lamotrigine.46 This study also found that phenytoin was associated with higher concentrations of bone-specific alkaline phosphatase and reduced concentrations of insulin growth factor-1 (IGF-1) compared to carbamazepine, valproate, and lamotrigine. Epilepsy itself has been found to negatively influence bone health. A study of biochemical markers of bone metabolism of children with self-limited epilepsy with centrotemporal spikes, which included an untreated group, concluded that epilepsy alone can affect many aspects of bone metabolism.45 Smoking and alcohol consumption, and a sedentary lifestyle are also more common among people with epilepsy, which can contribute to an increased risk of osteoporosis.
The combined risks associated with oestrogen deficiency after menopause, as well as earlier menopause associated with epilepsy leading to longer oestrogen deficiency, and the negative effects of epilepsy and ASMs on bone health all contribute to the risk of osteoporosis and fractures in postmenopausal females with epilepsy. In a study of 26 postmenopausal females with epilepsy, 62% had osteoporosis compared to 27% in a matched control group.47 It is crucial for clinicians to recognise that postmenopausal females with epilepsy are especially vulnerable to developing osteoporosis, as hip fractures are associated with a 1-year mortality rate of around 20%.43 However, evidence-based guidelines about the ideal screening and prevention strategies are lacking. Lifestyle factors that support bone health, such as adequate nutrition, weight-bearing exercise, and avoidance of smoking and alcohol, should be encouraged. Vitamin D and calcium supplementation should be encouraged, especially for those with an inadequate dietary intake and those at higher risk for osteoporosis.48 Routine screening of 25-hydroxyvitamin D should be considered once or twice per year, especially in those taking enzyme-inducing ASMs.49 Screening dual-energy X-ray absorptiometry scans should be considered in patients with epilepsy at elevated risk, including postmenopausal females.48 If osteoporosis is identified, then a referral to an endocrinologist or bone specialist should be considered for further management.
Other Comorbidities During Transition to Menopause
Sleep disturbances, a common symptom of perimenopause and menopause, may further exacerbate seizure risk. Vasomotor symptoms, such as night sweats and hot flushes, combined with chronic insomnia, contribute to sleep deprivation, a well-documented seizure trigger. Gómez-Santos et al.50 reported that menopausal females exhibit a loss of circadian robustness and worsening sleep quality compared to premenopausal females, which could negatively impact control.50 Interestingly, gabapentin has been shown to reduce vasomotor symptoms in menopausal females. There is evidence that gabapentin improves sleep quality and reduces hot flushes, which could potentially improve seizure control in females with epilepsy during menopause.51,52 These findings highlight the dual benefits of gabapentin for managing both seizures and menopausal symptoms.
The decline in oestrogen levels during menopause can exacerbate metabolic changes and increase the risk of insulin resistance, Type 2 diabetes, dyslipidaemia, and coronary artery atherosclerosis.53,54 This can disproportionately affect females with epilepsy who are at risk for earlier menopause. An earlier age of menopause also affects cardiovascular mortality.55 A recent cohort study found that epilepsy was associated with a higher risk of diabetes and adverse post-diabetes outcomes, such as infections and septicaemia.56 The clustering of comorbidities in menopause should prompt more effective risk factor management in females with epilepsy. ASMs such as valproate can be associated with metabolic disturbances, weight gain, and insulin resistance, in addition to elevating androgen levels, leading to hyperandrogenism and associated metabolic issues.57 Balancing and targeting other comorbidities during the transition to menopause in females with epilepsy can improve overall quality of life in this population.
DISCUSSION
While hormonal shifts during menstrual cycles and pregnancy offer insight into how the menopause continuum may affect seizure control in females with epilepsy, specific data on this topic are scant. No longitudinal studies have followed individuals with epilepsy through the menopausal continuum, resulting in significant knowledge gaps and a lack of evidence-based practice guidelines for managing epilepsy through the menopausal continuum.
When considering the influence of perimenopause and menopause on seizure control, current data are limited to small studies, primarily reliant on questionnaires. While anecdotal evidence suggests that seizure control may worsen during perimenopause secondary to unpredictable hormonal fluctuations, and improve with lower oestrogen concentrations in postmenopause, longitudinal studies are necessary to accurately determine this relationship. Furthermore, the contribution from endogenous hormonal fluctuations and medication clearance changes should be further examined. These studies would require following females with epilepsy from their reproductive years through the menopause continuum. Ideally, they would track seizure control; menstrual cycle patterns; follicular-stimulating hormone, anti-Müllerian hormone, and oestrogen concentrations; and ASM concentrations. With advancing technology and the ability to quantitatively track seizure frequency, such as the Responsive Neurostimulation System (RNS®; NeuroPace, Mountain View, California, USA), there may be future opportunities to objectively track seizures through the menopause transition. Longitudinal studies would also provide further data about the risks of early menopause in females with epilepsy, as current data from small studies suggest there is an elevated risk, potentially impacting seizure control. Systematically integrating data collection on a patient’s reproductive state may also further inform whether menopause (and not age alone) could be an important factor for surgical outcomes, especially as epilepsy surgery can be efficacious and safe in older populations (age: ≥50 years).58
Another important aspect of management that requires further study over the menopause continuum is the change in ASM metabolism and its impact on clinical care. For example, evidence supports that critical changes in metabolism occur with ageing for lamotrigine. With reduced clearance in postmenopausal years, lamotrigine concentrations may rise and lead to an increased risk of toxicity. The influence of hormonal changes at menopause on other ASMs also requires further study. Therapeutic drug monitoring has been instrumental during pregnancy in understanding clearance changes and optimising clinical care; a comparable approach may be beneficial during the menopausal continuum, with strategic adjustments for individual timelines.
MHT is the main treatment for bothersome menopause symptoms, such as vasomotor symptoms. Moreover, MHT may have long-term health benefits for people with early menopause. However, the one clinical trial studying MHT in epilepsy demonstrated worsened seizure frequency in a dose-dependent manner.14 Since this trial, the knowledge and use of MHT have greatly evolved, with a better understanding of the safety, efficacy, and ideal doses and routes of administration. Many MHTs have not been studied in females with epilepsy. Equally important, the effects of menopause symptoms and the consequences of leaving them untreated on seizure control remain unknown. Randomised, placebo-controlled trials are needed to evaluate the safety and efficacy of various MHTs, with attention to both seizure control and menopause symptom management.
Evidence demonstrates that postmenopausal females with epilepsy face an elevated risk of osteoporosis and fractures. Epilepsy itself, ASMs, and low oestrogen levels after menopause are established contributors to bone density loss. Despite these known risk factors, optimal screening protocols, including timing and frequency of bone mineral density assessments, and follow-up strategies for patients without baseline osteoporosis have yet to be defined. Although lifestyle interventions to preserve bone health are advocated, evidence-based approaches to mitigate fracture risk in this population with epilepsy are lacking.
Although not discussed in detail here, several medical conditions beyond osteoporosis, many of which are already comorbid with epilepsy, may experience a ‘second hit’ due to physiological changes at menopause, potentially accelerating their progression in people with epilepsy compared to those without. Particularly important for both quality of life and mortality are sleep disorders, cognitive decline, and cardiovascular disease.
In conclusion, perimenopause and menopause represent a crucial and significant transition in every female’s life. Evolving research on perimenopause and menopause may be applied to the postmenopause stage, with potential opportunities for preventative models of care. Several important considerations arise for people with epilepsy in this transitional state, including the influence of hormonal changes on seizure control, alterations to ASM metabolism, the effects of MHTs on epilepsy, and bone health. There are currently no practical guidelines to guide clinical care. This review highlights the imperative need for further research, particularly longitudinal studies, to advance understanding and improve the management and quality of life for midlife females with epilepsy.







