Authors: *Andrew Dugue1
1. Department of Neurology, NYU Grossman School of Medicine, New York City, USA
*Correspondence to [email protected]
Disclosure: The author has declared no conflicts of interest.
Keywords: Biomarkers, central vein sign, cerebrospinal fluid, diagnosis, McDonald criteria 2024, MRI, multiple sclerosis (MS), optic nerve, optical coherence tomography.
Citation: Neurol AMJ. 2025;2[1]:26-29. https://doi.org/10.33590/neurolamj/ZSFM1010
![]()
UPDATING THE FRAMEWORK FOR MULTIPLE SCLEROSIS DIAGNOSIS
In the Fall of 2023, a group of international experts convened to examine the McDonald criteria for the diagnosis of MS. There was an imperative for revision of the 2017 criteria, given the substantive MS research, particularly involving paraclinical testing and biomarkers, that had since emerged. They utilized a modified nominal group technique, requiring agreement of 80%, on statements and recommendations deemed important in the diagnosis of MS.1 According to Aaron Miller, Icahn School of Medicine at Mount Sinai, New York, USA, these statements and recommendations were derived from the past year of presentations and evidence from the National Multiple Sclerosis Society and European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).1 Over 70 questions were discussed and voted upon, culminating in the inclusion of new parameters to increase the sensitivity of the McDonald criteria. These parameters added to the definitions of dissemination in time and dissemination in space. A diagnostic algorithm based on the proposed criteria is provided in Figure 1.1
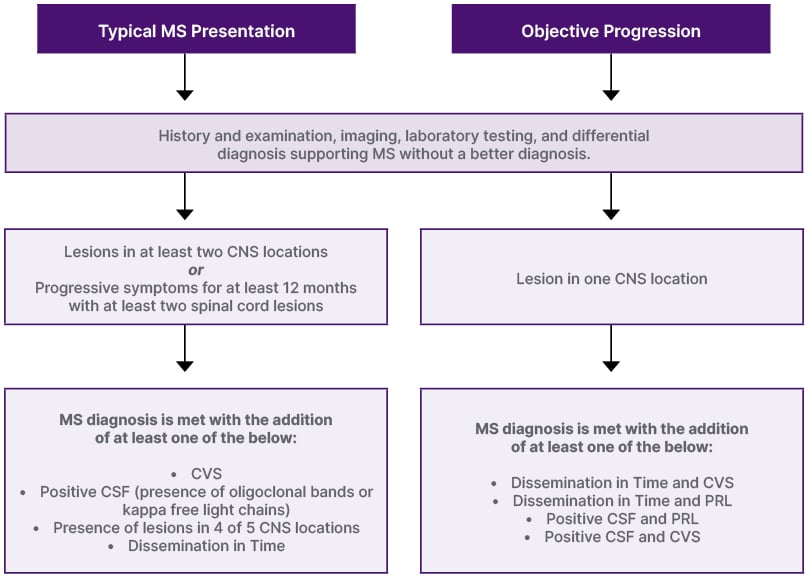
Figure 1: Diagnostic algorithm for multiple sclerosis diagnosis.*
*Diagnostic algorithm is adapted from Miller et al., 20251
CNS: central nervous system; CVS: central vein sign; CSF: cerebrospinal fluid; MS: multiple sclerosis; PRL: paramagnetic rim lesion.
WHAT’S NEW IN THE 2024 CRITERIA
Miller stated that the goal of the McDonald criteria is to facilitate the early diagnosis of MS, and that they were designed to be applied to only typical cases of MS.1 For example, unilateral optic neuritis, focal supratentorial syndrome, focal brainstem or cerebellar syndrome, or a partial myelopathy. He cautioned that they were not designed to be applied to atypical presentations, such as bilateral optic neuritis, complete ophthalmoplegia, complete transverse myelopathy, encephalopathy, headache, isolated fatigue, dizziness, or isolated vertigo.1
THE ROLE OF IMAGING AND BIOMARKERS
Laura Balcer, NYU Grossman School of Medicine, New York, USA, introduced the optic nerve as the fifth topographic site for MS lesions in the 2024 Revised McDonald criteria (Table 1). Evidence of a symptomatic or asymptomatic optic nerve lesion can be determined through paraclinical testing with orbital MRI, optical coherence tomography (OCT), and/or visual evoked potentials (VEP) (Table 2).1 Orbital MRI findings of optic nerve injury, through gadolinium enhancement and/or T2 hyperintensity, will be included in the same manner as other topographic sites. It was stressed that in order to make an accurate diagnosis, these findings must be interpreted in the correct clinical context, such as during an acute episode of optic neuritis with classic findings (eye pain, relative afferent pupillary defect, and dyschromatopsia).1 Work by Rachel Kenney, NYU Grossman School of Medicine, on OCT showed that an inter-eye asymmetry in retinal nerve fiber layer (RNFL) thickness and/or ganglion cell layer (GCL) thickness could distinguish eyes with prior optic neuritis amongst patients with MS.2 VEP demonstration of delayed latency could also indicate an optic nerve lesion. Steven Galetta, NYU Grossman School of Medicine, noted that studies by Brownlee, Bsteh, and Vidal-Jordana using the optic nerve as a fifth site with these paraclinical tests maintained McDonald criteria accuracy, while increasing their sensitivity.3-6 He stated that one cannot make the diagnosis of MS using the optic nerve as a fifth site without the correct clinical context. He noted that there are other causes of optic nerve abnormalities, such as glaucoma, a highly myopic eye, and maculopathies that can confound paraclinical testing.3 He also remarked that these tests (VEP, OCT, MRI) had been verified in well-characterized cohorts of patients, underscoring the importance of applying the criteria to those with a typical MS presentation.3
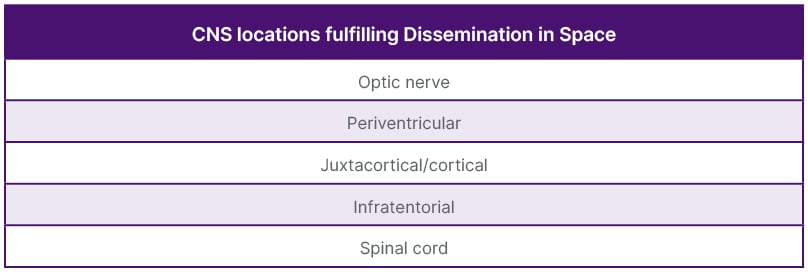
Table 1: Topographic locations for multiple sclerosis diagnosis.
CNS: central nervous system.
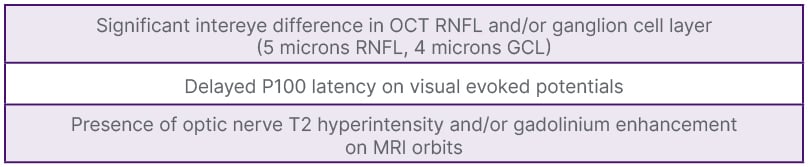
Table 2: Paraclinical test criteria for optic nerve lesions.*
*Official retinal nerve fiber layer and ganglion cell layer intereye difference measurement to be announced in the upcoming McDonald Criteria 2024 publication. The numbers provided in the Figure are based on research by Kenney et al.2
GCL: ganglion cell layer; OCT: optical coherence tomography; RNFL: retinal nerve fiber layer.
Balcer discussed cerebrospinal fluid (CSF) biomarkers in the 2024 McDonald criteria. She stated that the kappa-free light chain index is reflective of intrathecal B cell activity and is increased in MS1 It was added to the new McDonald criteria as a marker fulfilling dissemination in time. This marks the addition of a second CSF biomarker, aside from oligoclonal bands, fulfilling dissemination in time criteria. It was noted that there is approximately 87% concordance between the kappa-free light chain index and oligoclonal bands.1
APPLYING THE CRITERIA IN CLINICAL PRACTICE
Jiwon Oh, University of Toronto, Canada, reviewed the MRI findings in the 2024 criteria and remarked that this is the first time in McDonald criteria history that MRI has been incorporated beyond simply detecting new lesions. Two MRI findings were added: the central vein sign (CVS) and paramagnetic rim lesions (PRL). The CVS, a line or dot centrally located within a lesion on susceptibility sequences, reflects the pathologic mechanism of MS (perivenular inflammation and demyelination). The CVS’ sensitivity and specificity for MS is greater than 90%. It can be applied through the Select 6 and Rule of 6 rating methods. The Select 6 method supports MS if at least 6 lesions have central veins, while the Rule of 6 is used if there are fewer than 6 lesions and supports a diagnosis of MS if the majority of lesions have a central vein. PRLs, lesions from inflammation and demyelination related to paramagnetic effects of iron-laden microglia and macrophages at the lesion edge, are regarded as a marker of MS disease progression and confer over 90% specificity for MS.1 Shamik Bhattacharyya, Brigham and Women’s Hospital, Boston, Massachusetts, USA, cautioned that in periventricular lesions, there is a high rate of positive CVS even in people with non-MS pathologies. He noted that a combination of susceptibility and T2 weighted images allows for the best visualization of the CVS, particularly T2* or FLAIR* imaging.7 Critically, these imaging signs facilitate earlier diagnosis in patients across the spectrum of MS, particularly in those with radiologically isolated syndrome. The high specificity of these signs was noted to act as guard rails against MS misdiagnosis. Oh again emphasized that the new criteria should be used in those presenting with typical clinical syndromes, and that adherence to strict definitions of characteristic lesion topographies is important to minimize misdiagnosis.1