Abstract
Neuroendocrine tumors (NET) of the gallbladder represent an exceedingly rare entity, accounting for approximately 0.2% of all NETs and 2% of gallbladder malignancies. The authors present the case of a 27-year-old woman who presented with a 1-month history of upper abdominal pain and vomiting. Contrast-enhanced CT scan revealed a large gallbladder mass with homogeneous enhancement and extensive lymphadenopathy. Histopathological examination and immunohistochemistry confirmed a high-grade (Grade 3) NET with a Ki-67 proliferation index of 90%. This case highlights the diagnostic challenges associated with these rare tumours, emphasizing the critical role of multimodal diagnostic approaches, including radiological imaging, histopathology, and immunohistochemistry. The authors provide a comprehensive review of the literature on gallbladder NETs, discussing their epidemiology, pathogenesis, clinical presentation, diagnostic workup, treatment strategies, and prognostic factors. Early recognition and appropriate management of these aggressive tumors are crucial, as high-grade gallbladder NETs are associated with poor prognosis and high mortality rates.
Key Points
1. The pathogenesis of gallbladder neuroendocrine tumors remains poorly understood, with several competing theories, including intestinal metaplasia secondary to chronic inflammation, multipotent stem cell differentiation, neuroectodermal origin, and heterotopic pancreatic tissue, highlighting a significant knowledge gap in this rare disease.
2. Clinical presentation of gallbladder neuroendocrine tumors is typically nonspecific (right upper quadrant pain, nausea, vomiting) and resembles other gallbladder pathologies, with carcinoid syndrome being exceedingly rare (<1% of cases) due to the liver’s efficient metabolism of vasoactive substances.
3. The 2019 WHO classification system for neuroendocrine neoplasms emphasises both differentiation status (well versus poorly differentiated) and grade (based on Ki-67 index), which significantly impacts treatment decisions. Well-differentiated Grade 1/Grade 2 tumors potentially respond to somatostatin analogues and targeted therapies, whereas high-grade tumors require more aggressive approaches, such as platinum-based chemotherapy.
INTRODUCTION
Neuroendocrine tumors (NET) constitute a heterogeneous group of neoplasms that originate from cells of the diffuse neuroendocrine system.1 These tumors most commonly arise in the gastrointestinal tract (73%) and bronchopulmonary system (25.1%), with the remainder occurring in various other anatomical locations.2,3 NETs of the gallbladder are exceptionally rare, representing approximately 0.2–0.5% of all NETs and 2–2.1% of all gallbladder malignancies.4,2
The pathogenesis of gallbladder NETs remains incompletely understood. Unlike other parts of the gastrointestinal tract, the normal gallbladder mucosa does not contain neuroendocrine cells.5 Several theories have been proposed to explain the development of these tumors, including derivation from multipotent stem cells or neuroendocrine cells that arise in the setting of intestinal metaplasia secondary to chronic inflammation, most commonly associated with cholelithiasis.4,5
Gallbladder NETs typically present with nonspecific symptoms, including right upper quadrant abdominal pain, nausea, vomiting, and weight loss, which are similar to those of other gallbladder pathologies.6 Carcinoid syndrome, characterised by flushing, diarrhea, and bronchospasm, is exceedingly rare in gallbladder NETs due to the efficient metabolism of serotonin and other vasoactive substances by the liver before they reach the systemic circulation.3
The diagnosis of gallbladder NETs is challenging due to their nonspecific clinical presentation and imaging features. These tumors are often discovered incidentally during cholecystectomy for presumed benign gallbladder disease or during imaging studies performed for unrelated indications.4,6 Radiological imaging, particularly ultrasonography, CT, and MRI, plays a crucial role in the initial detection and staging of these tumors. However, definitive diagnosis requires histopathological examination and immunohistochemical confirmation.7
According to the 2019 WHO classification, NETs are categorised based on their differentiation (well-differentiated versus poorly differentiated) and grade (Grade 1, Grade 2, or Grade 3), which is determined by the mitotic count and Ki-67 proliferation index.8 Well-differentiated NETs are further classified as Grade 1 (Ki-67: ≤3%), Grade 2 (Ki-67: 3–20%), or Grade 3 (Ki-67: >20%), while poorly differentiated neuroendocrine carcinomas (NEC) are always classified as Grade 3 (typically with Ki-67: >55%).8,9
The management of gallbladder NETs depends on the tumor stage, grade, and patient-specific factors. Surgical resection remains the mainstay of treatment for localized disease, while systemic therapy, including chemotherapy and targeted agents, is employed for advanced or metastatic disease.10,11 The prognosis for patients with gallbladder NETs varies significantly based on the tumor grade and stage at diagnosis, with high-grade tumors associated with poor outcomes.4,6In this article, the authors present a rare case of a high-grade neuroendocrine tumor of the gallbladder in a young female patient, highlighting the diagnostic challenges and management considerations associated with these uncommon neoplasms. Additionally, the authors provide a comprehensive review of the literature on gallbladder NETs, discussing their epidemiology, pathogenesis, clinical presentation, diagnostic workup, treatment strategies, and prognostic factors.
CASE PRESENTATION
A 27-year-old woman with no significant past medical history presented to the authors’ institution with a 1-month history of insidious, non-progressive upper abdominal pain accompanied by intermittent episodes of vomiting. The patient reported no specific aggravating or relieving factors for her symptoms. She denied jaundice, fever, weight loss, or changes in bowel habits. There was no family history of malignancy.
Physical examination revealed a young woman in no acute distress. Vital signs were within normal limits. Abdominal examination demonstrated mild tenderness in the right upper quadrant without rebound or guarding. No palpable masses or hepatomegaly were appreciated. There was no evidence of jaundice, and the remainder of the physical examination was unremarkable.
Laboratory studies, including complete blood count, liver function tests, and serum tumor markers (carcinoembryonic antigen [CEA] and cancer antigen [CA] 19–9), were within normal limits. Serum chromogranin A (CgA) and 5-hydroxyindoleacetic acid (5-HIAA) levels were not initially measured, as a neuroendocrine tumor was not part of the initial differential diagnosis.
Based on the clinical presentation, abdominal ultrasonography was performed, which revealed a heterogeneous mass in the gallbladder with associated regional lymphadenopathy. Subsequently, a contrast-enhanced CT scan of the abdomen was obtained for further characterisation of the lesion.
The non-contrast CT demonstrated a large, homogeneously iso-dense mass arising from the gallbladder, measuring approximately 6.8×8.7×7.4 cm (craniocaudal × anteroposterior × transverse) on unenhanced images.
Following contrast administration, the mass exhibited homogeneous enhancement in both the arterial and venous phases. Notably, there was mild irregular wall thickening at the fundus of the gallbladder. Additionally, multiple enlarged, conglomerated lymph nodes with homogeneous enhancement were identified in the periportal, para-aortic, aortocaval, retrocaval, retro-aortic, and infrarenal regions. The largest lymph node conglomerate, measuring 6.5×4.1 cm, was located in the pre- and para-aortic region. There was no evidence of distant metastasis to the liver or other organs. These imaging findings were suggestive of a malignant gallbladder neoplasm, with differential diagnoses including adenocarcinoma, lymphoma, and, less commonly, neuroendocrine tumor.
Given the extensive lymphadenopathy and the size of the primary lesion, an ultrasound-guided percutaneous biopsy of the gallbladder mass was performed to establish a tissue diagnosis and guide further management. Histopathological examination of the biopsy specimen revealed a poorly differentiated neoplasm composed of sheets of small to medium-sized cells with hyperchromatic nuclei, scant cytoplasm, and frequent mitotic figures.
Immunohistochemical staining was performed to further characterize the neoplasm. The tumor cells demonstrated focal expression of cytokeratin (CK) and strong, diffuse positivity for neuroendocrine markers, including synaptophysin and neural cell adhesion molecule (CD56). The Ki-67 proliferation index was exceptionally high, approaching 90% in hotspot areas. These findings were consistent with a high-grade (Grade 3) neuroendocrine tumor of the gallbladder.
Following the diagnosis, additional laboratory tests were performed, including serum chromogranin A, which was markedly elevated at 890 ng/mL (reference range: 0–100 ng/mL). A somatostatin receptor scintigraphy (Octreoscan) was also conducted, which demonstrated intense uptake in the gallbladder mass and multiple lymph node regions, confirming the presence of somatostatin receptors and the neuroendocrine nature of the tumor.
Based on the clinical, radiological, and pathological findings, the patient was diagnosed with a Stage IV (T3N1M0) high-grade neuroendocrine tumor of the gallbladder according to the American Joint Committee on Cancer (AJCC) staging system for gallbladder cancer. Given the advanced stage and high-grade nature of the tumor, a multidisciplinary tumor board discussion was held to determine the optimal management strategy.
Considering the extensive lymphadenopathy and locally advanced nature of the disease, neoadjuvant chemotherapy with a platinum-based regimen (cisplatin and etoposide) was recommended, followed by reassessment for potential surgical resection. The patient underwent this treatment regimen, with periodic clinical and radiological evaluations to assess response.
Following the introduction of neoadjuvant chemotherapy with etoposide and cisplatin, the patient had an excellent clinical and radiological response. Following four cycles of chemotherapy over 12 weeks, follow-up contrast-enhanced CT scans showed a marked reduction in the mass of the primary gallbladder tumor (from 6.8×8.7×7.4 cm to 4.2×5.1×4.8 cm), as well as in the diffuse lymphadenopathy, with the largest para-aortic lymph node conglomerate reducing from 6.5×4.1 cm to 3.2×2.8 cm. Biochemical response was also impressive, with serum chromogranin A levels falling from a baseline of 890 ng/mL to 245 ng/mL (a 72% fall).
The patient tolerated chemotherapy well, with minimal Grade 1–2 toxicities such as fatigue, nausea, and reversible neutropenia; these were managed successfully with supportive care. During the treatment period, her performance status remained excellent (Eastern Cooperative Oncology Group [ECOG]: 0–1), with good relief of her initial symptoms, which were vomiting and abdominal pain. Based on this good response, the multidisciplinary team recommended proceeding with surgical resection. Thus, the patient was successfully treated with a radical cholecystectomy with regional lymphadenectomy and resection of segments IVb and V of the liver. Histopathological examination of the resected specimen showed intense treatment-related changes with extensive necrosis and fibrosis, with scattered viable tumor cells only. A complete resection was achieved with free margins (R0).
At 18 months post-surgery, the patient remains disease-free, with normal serum chromogranin A (CgA) levels (42 ng/mL) and no disease recurrence on follow-up imaging. She is back to normal activities with excellent quality of life, with the outcome demonstrating that, with early detection, good multimodal treatment, and total surgical resection, even in high-grade gallbladder NETs, a good outcome is possible. The case emphasizes the role of neoadjuvant chemotherapy in advanced disease, as it may enable curative resection in selected patients.
DISCUSSION
Epidemiology and Risk Factors
Gallbladder NETs are exceedingly rare, accounting for approximately 0.2–0.5% of all NETs and 2–2.1% of all gallbladder malignancies.2,4 According to the Surveillance, Epidemiology, and End Results (SEER) database analysis by Mahendraraj et al.,12 only 278 cases of gallbladder NETs were reported in the United States between 1973–2012, representing 0.5% of all NETs and 0.2% of all gallbladder cancers.
These tumors predominantly affect females, with a female to male ratio of approximately 2.1:1.12 The median age at diagnosis is reported to be 64 years, with most cases occurring in the sixth and seventh decades of life.6,12 Our patient’s young age (27 years) at presentation makes this case particularly unusual, as gallbladder NETs are exceedingly rare in individuals younger than 40 years (Table 1).
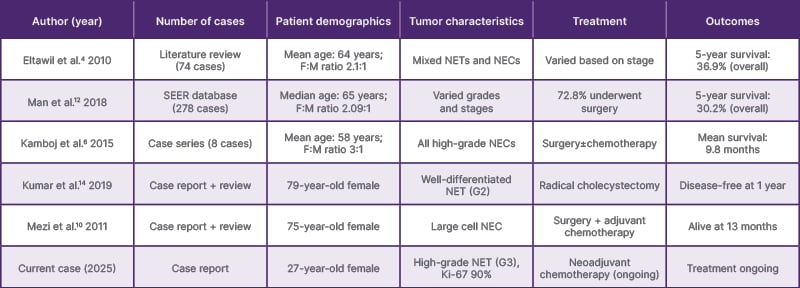
Table 1: Summary of published case reports and series of gallbladder neuroendocrine tumors (selected studies).
F:M: Female to male; G2: Grade 2; G3: Grade 3; NEC: neuroendocrine carcinoma; NET: neuroendocrine tumor; SEER: Surveillance, Epidemiology, and End Results.
The risk factors for gallbladder NETs are not well established due to the rarity of these tumors. However, chronic inflammation associated with cholelithiasis has been implicated in the pathogenesis of gallbladder NETs, similar to adenocarcinoma of the gallbladder.4,5 Other potential risk factors, such as obesity, diabetes, and exposure to carcinogens, which are known risk factors for conventional gallbladder adenocarcinoma, may also play a role in the development of gallbladder NETs, but evidence is limited.6
Pathogenesis
The pathogenesis of gallbladder NETs remains incompletely understood, primarily due to the absence of neuroendocrine cells in the normal gallbladder mucosa. Several theories have been proposed to explain the development of these tumors.4,5:
- Intestinal metaplasia theory:
chronic inflammation, most commonly associated with cholelithiasis, can lead to intestinal metaplasia of the gallbladder mucosa. This metaplastic epithelium may contain neuroendocrine cell that can subsequently undergo neoplastic transformation. - Multipotent stem cell theory:
gallbladder NETs may arise from multipotent stem cells that have the capacity to differentiate along neuroendocrine lineages. - Neuroectodermal origin theory:
some researchers have suggested that gallbladder NETs might originate from neuroectodermal cells that migrate to the gallbladder during embryological development. - Heterotopic pancreatic tissue theory: rarely, heterotopic pancreatic tissue in the gallbladder wall may give rise to NETs.
The molecular pathogenesis of gallbladder NETs is poorly characterized. Limited studies have reported alterations in various oncogenes and tumor suppressor genes, including TP53, KRAS, and SMAD4, particularly in high-grade neuroendocrine carcinomas.13 However, comprehensive genomic profiling of these rare tumors is lacking, and further research is needed to elucidate the molecular mechanisms underlying their development and progression.
CLINICAL PRESENTATION
The clinical presentation of gallbladder NETs is typically nonspecific and similar to that of other gallbladder pathologies, including cholecystitis, cholelithiasis, and adenocarcinoma.4,6 Common symptoms include right upper quadrant abdominal pain, nausea, vomiting, anorexia, and weight loss. Jaundice may occur in advanced disease due to biliary obstruction by the primary tumor or metastatic lymph nodes.
Carcinoid syndrome, characterized by flushing, diarrhea, bronchospasm, and right-sided valvular heart disease, is exceedingly rare in gallbladder NETs, occurring in <1% of cases.3 This is attributed to the efficient metabolism of serotonin and other vasoactive substances by the liver before they reach the systemic circulation. When carcinoid syndrome does occur, it is typically associated with extensive liver metastases that allow these substances to bypass hepatic metabolism and enter the systemic circulation.3,4
In the authors’ case, the patient presented with nonspecific symptoms of upper abdominal pain and vomiting, without evidence of jaundice or carcinoid syndrome, despite having an advanced-stage tumor with extensive lymphadenopathy. This highlights the often insidious nature of these tumors and the challenges associated with early diagnosis.
Diagnosis and Radiological Features
The diagnosis of gallbladder NETs is challenging due to their nonspecific clinical presentation and imaging features. These tumors are often discovered incidentally during cholecystectomy for presumed benign gallbladder disease or during imaging studies performed for unrelated indications.4,6
Radiological Imaging
Ultrasonography is typically the initial imaging modality employed in patients with suspected gallbladder pathology. Gallbladder NETs may appear as polypoid lesions, wall thickening, or intraluminal masses on ultrasound. However, these findings are nonspecific and can be seen in various gallbladder conditions, including adenocarcinoma, adenomatous polyps, and inflammatory processes.7,14
CT and MRI provide more detailed information about the primary tumor, regional lymphadenopathy, and potential distant metastases. On CT, gallbladder NETs typically appear as enhancing masses, often with heterogeneous enhancement patterns due to areas of necrosis or hemorrhage.14 In the authors’ case, the tumor demonstrated homogeneous enhancement in both arterial and venous phases, which is an atypical finding for NETs but may be seen in some cases, particularly high-grade tumors with rapid growth and less necrosis.
Functional imaging modalities, such as somatostatin receptor scintigraphy (indium In-111 pentetreotide) and gallium-68 (68 Ga)-DOTATATE PET/CT, are valuable tools for the diagnosis and staging of NETs. These techniques rely on the expression of somatostatin receptors (SSTR) on the surface of neuroendocrine tumor cells.11,15 However, high-grade NETs may have reduced SSTR expression, potentially limiting the utility of these modalities in such cases. Nevertheless, in the authors’ patient, indium In-111 pentetreotide demonstrated intense uptake in the gallbladder mass and lymph nodes, confirming the neuroendocrine nature of the tumor.
Histopathology and Immunohistochemistry
Gallbladder NETs are diagnosed through histopathology and confirmed by immunohistochemistry. The 2019 WHO classification categorizes NETs by differentiation (well versus poorly differentiated) and grade (1–3), based on mitotic rate and Ki-67 index.8,9
Well-differentiated NETs are characterized by an organoid growth pattern with uniform cells containing round to oval nuclei with stippled chromatin and moderate amounts of cytoplasm. These tumors express neuroendocrine markers, including synaptophysin, CgA, and CD56, and are further classified as Grade 1 (Ki-67: ≤3%), Grade 2 (Ki-67: 3-20%), or Grade 3 (Ki-67: >20%).8,9
Poorly differentiated NECs exhibit a sheet-like or diffuse growth pattern with cells demonstrating high nuclear-to-cytoplasmic ratios, hyperchromatic nuclei, inconspicuous nucleoli, and frequent mitotic figures. These tumors are always classified as Grade 3 (typically with Ki-67: >55%) and are further subtyped as small cell or large cell variants.8,9
In the authors’ case, the histopathological examination revealed a poorly differentiated neoplasm with a high mitotic rate, and immunohistochemistry demonstrated focal expression of cytokeratin and strong positivity for neuroendocrine markers (synaptophysin and CD56). The Ki-67 proliferation index was exceptionally high at 90%, consistent with a high-grade (Grade 3) neuroendocrine tumor. The distinction between a well-differentiated Grade 3 NET and a poorly differentiated Grade 3 NEC is important for therapeutic decision-making, as these entities may have different biological behaviors and responses to treatment8,1 (Table 2).
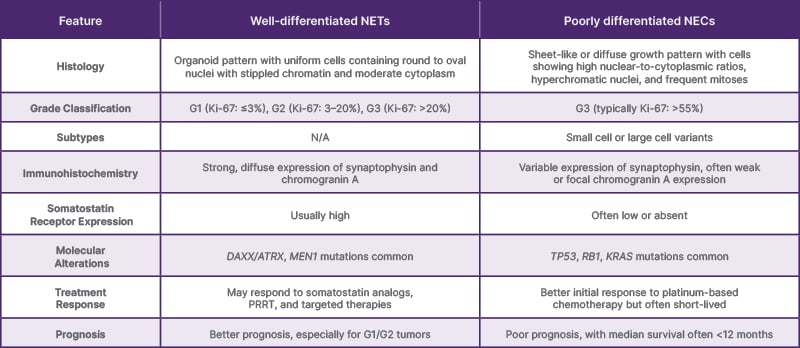
Table 2: Comparison of well-differentiated neuroendocrine tumors and poorly differentiated neuroendocrine carcinomas of the gallbladder.
G1: Grade 1; G2: Grade 2; G3: Grade 3; NEC: neuroendocrine carcinoma; NET: neuroendocrine tumor; PRRT: peptide receptor radionuclide therapy.
Serum Biomarkers
Serum biomarkers, such as CgA and neuron-specific enolase (NSE), can aid in the diagnosis and monitoring of NETs. CgA is a secretory protein stored in the neurosecretory granules of neuroendocrine cells, and is elevated in approximately 60–80% of patients with NETs.7,15 NSE is a glycolytic enzyme that is more commonly elevated in poorly differentiated NECs than in well-differentiated NETs. In the authors’ patient, serum CgA was markedly elevated, supporting the diagnosis of a neuroendocrine neoplasm.
Urinary 5-HIAA, a metabolite of serotonin, is another biomarker that can be used to diagnose and monitor carcinoid syndrome. However, its utility in gallbladder NETs is limited, as these tumors rarely produce serotonin or cause carcinoid syndrome.3
Treatment Strategies
The management of gallbladder NETs depends on the tumor stage, grade, and patient-specific factors (Table 3). A multidisciplinary approach involving surgeons, oncologists, radiologists, and pathologists is essential for optimal outcomes.4,7,8,11
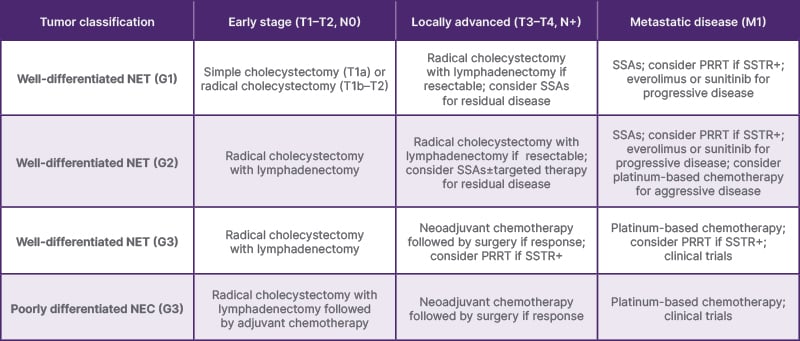
Table 3: Recommendations for the management of gallbladder neuroendocrine tumors based on tumor grade and stage.
G1: Grade 1; G2: Grade 2; G3: Grade 3; N: node; NET: neuroendocrine tumor; NEC: neuroendocrine carcinoma; PRRT: peptide receptor radionuclide therapy; SSA: somatostatin analog; SSTR+:somatostatin receptor-positive.
Surgical Management
Surgical resection remains the mainstay of treatment for localized gallbladder NETs. The extent of surgery depends on the tumor stage and location within the gallbladder.10,11 For early-stage tumors confined to the gallbladder mucosa or muscular layer (T1a or T1b), simple cholecystectomy may be adequate. However, for tumors extending beyond the muscular layer (T2 or higher) or with evidence of regional lymphadenopathy, radical cholecystectomy with hepatic resection and regional lymphadenectomy is recommended.10,11
In patients with advanced disease, such as the present case with extensive regional lymphadenopathy, neoadjuvant chemotherapy may be considered to downstage the tumor and potentially increase the likelihood of complete surgical resection. However, the efficacy of this approach in gallbladder NETs is not well-established due to the rarity of these tumors and the lack of prospective studies.10
Systemic Therapy
Systemic therapy plays a crucial role in the management of locally advanced, metastatic, or high-grade gallbladder NETs. The choice of systemic therapy depends on the tumor grade, differentiation, and extent of disease.10,11
For well-differentiated Grade 1 or Grade 2 NETs, somatostatin analogs (octreotide or lanreotide) are often used as first-line therapy, particularly in patients with SSTR-positive tumors. These agents have been shown to inhibit tumor growth and improve progression-free survival in patients with advanced NETs.10,16
For high-grade (Grade 3) tumors, particularly poorly differentiated NECs, platinum-based chemotherapy regimens, such as cisplatin or carboplatin combined with etoposide, are typically employed. These regimens have demonstrated response rates of 30–50% in high-grade gastrointestinal NECs, although responses are often short-lived.11,17 In the authors’ case, the patient was initiated on cisplatin and etoposide chemotherapy, given the high-grade nature of the tumor and extensive regional lymphadenopathy.
Targeted therapies, including mammalian targets of rapamycin (mTOR) inhibitors (everolimus) and tyrosine kinase inhibitors (TRK, sunitinib), have shown efficacy in advanced well-differentiated NETs. However, their role in high-grade NETs or NECs is less well-established.10,16
Peptide Receptor Radionuclide Therapy
Peptide receptor radionuclide therapy (PRRT) with radiolabelled somatostatin analogs, such as lutetium-177 (177Lu)-DOTATATE, is an emerging treatment modality for patients with advanced, SSTR-positive NETs. This therapy delivers targeted radiation to tumor cells expressing SSTRs, resulting in cell death. The NETTER-1 trial demonstrated significant improvements in progression-free survival and response rates with 177Lu-DOTATATE compared to high-dose octreotide long-acting repeatable in patients with advanced midgut NETs.18 However, the efficacy of PRRT in gallbladder NETs specifically, particularly high-grade tumors, is not well-established due to the rarity of these tumors and potential reduced SSTR expression in high-grade lesions.11,17
Prognosis and Prognostic Factors
The prognosis for patients with gallbladder NETs varies significantly based on the tumor grade, stage, and patient-specific factors. According to the SEER database analysis by Mahendraraj et al.,12 the overall 5-year survival rate for gallbladder NETs is approximately 30.2%, which is significantly lower than that for NETs in other anatomical locations.
High-grade (Grade 3) gallbladder NETs, particularly poorly differentiated NECs, are associated with a dismal prognosis, with reported 5-year survival rates of less than 10%.4,6 The aggressive nature of these tumors, coupled with their tendency for early lymphatic and distant metastasis, contributes to their poor outcomes.
Several factors have been identified as prognostic indicators in gallbladder NETs:6,12
- Tumor grade: high-grade tumors, particularly those with a Ki-67 proliferation index of >55%, are associated with worse outcomes compared to low-grade tumors.
- Tumor stage: advanced stage at diagnosis, including the presence of regional lymphadenopathy or distant metastases, is associated with poorer prognosis.
- Age at diagnosis: older age at diagnosis (>60 years) is associated with worse outcomes, potentially due to comorbidities and reduced tolerance to aggressive treatments.
- Surgical resection: complete surgical resection with negative margins is associated with improved survival, highlighting the importance of early diagnosis and appropriate surgical management.
In the present case, the patient’s young age (27 years) may be a favorable prognostic factor. However, the high-grade nature of the tumor (Ki-67 index of 90%) and the presence of extensive regional lymphadenopathy are associated with a poor prognosis. The response to neoadjuvant chemotherapy and the potential for subsequent surgical resection will be critical determinants of the patient’s long-term outcome.
CONCLUSION
Gallbladder NETs are rare malignancies with a wide spectrum of clinical presentations and biological behaviors. High-grade NETs, as observed in our case, pose significant diagnostic and therapeutic challenges due to their aggressive nature and poor prognosis. A comprehensive diagnostic approach, including radiological imaging, histopathological examination, and immunohistochemistry, is essential for accurate diagnosis and appropriate management.
The management of gallbladder NETs requires a multidisciplinary approach, with treatment strategies tailored to the individual patient based on tumor grade, stage, and specific patient factors. Surgical resection remains the cornerstone of treatment for localized disease, while systemic therapy, including chemotherapy and targeted agents, plays a crucial role in the management of advanced or high-grade tumors.
Despite advances in diagnostic modalities and therapeutic options, the prognosis for patients with high-grade gallbladder NETs remains poor, underscoring the need for early detection, accurate diagnosis, and optimal management. Further research is warranted to better understand the pathogenesis of these rare tumors and to develop more effective treatment strategies.







