Abstract
Triple-negative breast cancer (TNBC) remains the most aggressive subtype of breast cancer, with a higher risk of recurrence in the early-stage setting compared to other subtypes. While TNBC is defined as breast cancer that lacks estrogen receptor and progesterone receptor expression and is without human epidermal growth factor receptor 2 (HER2) overexpression, it is increasingly recognized as a very heterogeneous disease. Utilization of neoadjuvant chemotherapy (NAC), with or without immunotherapy (depending on the clinical stage), has significantly improved clinical outcomes in early-stage TNBC (particularly in Stage II and III disease). Use of NAC offers the opportunity to de-escalate surgical treatment and evaluate treatment response, allowing for improved prognostication and further tailoring of post-operative systemic therapy. However, there continues to be a need for the personalization of systemic therapy strategies according to recurrence risk. More effective systemic therapies are still needed for patients who have poor response to NAC. Conversely, there remains a need for the identification of appropriate candidates for systemic therapy de-escalation, particularly given the potentially life-altering toxicities of current chemo-immunotherapy strategies. In this review the authors outline the current neoadjuvant paradigm for early-stage TNBC and emerging therapeutic strategies in this challenging disease state, along with questions that remain unanswered in the field.
Key Points
1. Early-stage triple-negative breast cancer is a heterogenous breast cancer subtype. Adoption of neoadjuvant chemo-immunotherapy has resulted in significant improvements in clinical outcomes and has improved the ability to tailor adjuvant strategies.
2. Biomarkers to appropriately tailor systemic therapy strategies for patients at higher versus lower risk of recurrence and mortality remain critically needed.
3. Multiple novel therapeutics (particularly antibody–drug conjugates) that have shown efficacy in the metastatic setting are being evaluated in the neoadjuvant setting and in patients with residual disease after neoadjuvant chemotherapy, offering the potential to further improve outcomes.
INTRODUCTION
Triple-negative breast cancer (TNBC) comprises 10–15% of all breast cancers. It is a very heterogeneous disease, characterized by the absence of expression of estrogen receptors (ER) and progesterone receptors (PR), as well as a lack of human epidermal growth factor receptor 2 (HER2) overexpression.1,2 Early TNBC is managed primarily with neoadjuvant chemotherapy (NAC) in combination with immunotherapy for most patients with Stage II–III TNBC.3 Recently, an increased understanding of the molecular features and expression of biomarkers that either drive the biology or predict sensitivity to specific agents has informed the development of novel therapeutics currently used in the metastatic setting, many of which are being actively explored in the curative setting. Despite this progress, many challenges remain, chiefly the persistently higher recurrence risk in the early-stage setting compared to other breast cancer subtypes, and the growing but still limited treatment options in the metastatic setting.
With current NAC approaches, approximately 50–65% of patients with non-metastatic TNBC achieve pathologic complete response (pCR), which has consistently correlated with favorable long-term survival outcomes.4,5 Unlike hormone receptor-positive breast cancer, which is often associated with late recurrences, operable TNBC is characterized by early recurrences, typically within the first 3–5 years after initial treatment, with visceral disease (including lung, liver, and brain metastasis) being more common.6-8 Patients with locally advanced cancer, or with poor response to NAC, exhibit the highest rates of disease recurrence and mortality. Additional work is still needed to appropriately tailor systemic therapies and improve clinical outcomes while minimizing toxicities. Furthermore, better prognostic tools prior to and during NAC are needed to inform the selection and duration of therapeutic agents, both in the neoadjuvant and adjuvant setting. In this review, the authors summarize the current treatment paradigm for early-stage TNBC, with a focus on NAC, ongoing questions, and research efforts to further tailor systemic therapy strategies to the underlying recurrence risk.
EPIDEMIOLOGY AND CLINICAL FEATURES
TNBC can affect all women regardless of race, but it disproportionally affects women of African American, Hispanic, and Indian ancestry. Furthermore, it occurs at a higher frequency among younger women (<50 years old), and is more frequently associated with germline BRCA1, BRCA2, and PALB2 pathogenic mutations.9,10 While the rates of hormone receptor positive breast cancer are rising, there has been a slight decline in the incidence of TNBC since 2005.11 While not uniformly linked, some studies suggest that early menarche, late menopause, hormone therapy use, and alcohol consumption are associated with an increased risk of TNBC.12 TNBC can be mammographically occult, and sometimes manifests as an “interval cancer” (detected in between routine screening mammograms). Histologically, TNBC is typically characterized by high proliferation rates and higher tumor grades compared to other breast cancer subtypes.12
CURRENT STANDARD OF CARE FOR EARLY TRIPLE-NEGATIVE BREAST CANCER
NAC has become the preferred treatment approach for early-stage TNBC for several reasons, including: 1) facilitation of assessment of response (e.g., calculation of residual cancer burden [RCB]), which has implications on long-term outcomes and allows the tailoring of subsequent therapies according to the recurrence risk; 2) disease downstaging allowing de-escalation of breast and axillary surgery; and 3) the use of pCR as a preliminary efficacy endpoint, accelerating drug development.13,14 Figure 1 shows a proposed algorithm for the approach to early-stage TNBC in 2025.
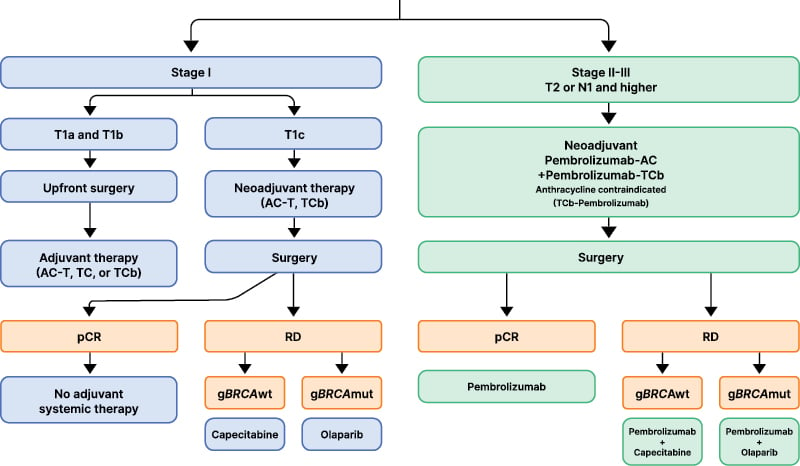
Figure 1: Suggested algorithm for early triple-negative breast cancer in 2025.
Created with BioRender® (Toronto, Canada).
AC: anthracycline and cyclophosphamide; AC-T: anthracycline and cyclophosphamide followed by paclitaxel; gBRCA
wt: germline BRCA wildtype; gBRCAmut: germline BRCA mutated; pCR: pathologic complete response; RD: residual disease; TC: docetaxel and cyclophosphamide; TCb: carboplatin and paclitaxel.
Stage I
Patients with clinical Stage T1a (2–5 mm) or T1b (6–10 mm) node negative (N0) TNBC are typically treated with upfront surgery. The administration of adjuvant chemotherapy for these populations remains controversial, given that most clinical trials evaluating its benefits have either excluded or enrolled very small numbers of people with Stage I TNBC. Controversies notwithstanding, adjuvant chemotherapy is typically recommended for T1b, and omitted for T1a tumors.3 In a recent Surveillance, Epidemiology, and End Results analysis, which included over 8,000 patients with Stage I TNBC treated between 2010–2019, 71% of those with T1c, 25% of those with T1b, and 20% of those with T1a and T1mi TNBC received adjuvant chemotherapy.15 Chemotherapy utilization has significantly increased over time in the USA, particularly for T1bN0 and T1cN0 tumors. Breast cancer-specific survival was excellent, regardless of chemotherapy administration for most patients with T1a-b N0 TNBC, with a chemotherapy benefit in breast cancer-specific survival observed only in the T1cN0 TNBC subset.15
The choice of a specific regimen for Stage I TNBC is also controversial, with anthracycline- and taxane-based regimens still considered the standard of care,16 particularly for T1cN0 TNBC. However, taxane-based regimens with the omission of anthracycline (i.e., docetaxel plus cyclophosphamide, or taxane plus carboplatin) have also shown favorable efficacy and safety, particularly in N0 TNBC.17,18 The pooled analysis of the ABC trials evaluated the efficacy of anthracycline (anthracycline, cyclophosphamide, and taxane) versus non-anthracycline containing regimens (docetaxel and cyclophosphamide for 6 cycles) in patients with early-stage HER2-negative breast cancer. While ultimately, the anthracycline-containing regimens were superior, the absolute benefit in patients with N0 TNBC was small.19 In other studies that are not specifically focused on Stage I TNBC, platinum and taxane-containing regimens without an anthracycline have been associated with favorable pCR rates in the neoadjuvant setting, and with improved survival, both in the neoadjuvant and adjuvant settings.18,20 In the WSG-ADAPT-TN trial, carboplatin plus nab-paclitaxel resulted in a pCR rate of 46%, and in another study evaluating two cohorts, carboplatin plus docetaxel yielded pCR rates of 55%, with RCB 0–I rates of 68%.20,21 Prospective data specifically tailoring systemic therapy intensity for patients with Stage I TNBC are needed.
Stage II–III
In patients with tumors ≥2 cm or node positive TNBC, chemo-immunotherapy following the KEYNOTE-522 regimen has become the standard of care.22-24 This randomized double-blind clinical trial enrolled patients with previously untreated early-stage TNBC that was either T1c N1-2 or T2-4 N0-2. Patients were randomly assigned in a 2:1 fashion to receive pembrolizumab versus placebo with carboplatin and paclitaxel for 12 weeks, followed by pembrolizumab versus placebo with anthracycline and cyclophosphamide for 12 weeks, which was followed by surgery. In the adjuvant setting, patients continued either pembrolizumab or placebo for an additional 27 weeks (9 cycles), according to their prior randomization assignment. Importantly, the achievement of pCR or lack thereof was not considered in the assignment of the adjuvant therapy strategy. Furthermore, patients who were not achieving pCR in KEYNOTE-522 did not receive adjuvant capecitabine (which subsequently became the standard of care).25 Ultimately, KEYNOTE-522 showed that the addition of pembrolizumab to NAC resulted in a 7% improvement in pCR, with a pCR rate of 63% in the chemo-immunotherapy group, compared to 56% with chemotherapy alone.24 In addition, the incorporation of pembrolizumab was associated with significant improvements in 5-year event-free survival (EFS; 81% versus 72%; hazard ratio [HR]: 0.65; 95% CI: 0.51–0.83) and in 5-year overall survival (OS; 87% versus 82%; HR: 0.66; 95% CI: 0.50–0.87) compared to chemotherapy alone.23,24 Importantly, patients with residual disease appeared to derive a more notable benefit, with that subset demonstrating a 5-year OS of 72% with pembrolizumab versus 66% without (HR: 0.76; 95% CI: 0.56–1.05).26 Unlike in the metastatic setting, the benefit derived with immunotherapy was independent of programmed death-ligand 1 (PD-L1) status and several other exploratory biomarkers.27
Several large, randomized trials have tested the addition of immunotherapy agents other than pembrolizumab to NAC in early-stage TNBC.28-30 IMpassion031 evaluated the addition of pre- and post-operative atezolizumab to NAC, which led to a significant improvement in pCR (58% versus 41%; absolute difference: 17%; p=0.0044). The 2-year EFS was numerically improved, but without reaching statistical significance (85% versus 80%; HR: 0.76; 95% CI: 0.47–1.21).31 Similarly, GeparNuevo evaluated the addition of pre-operative durvalumab only to NAC. In this study, despite pCR numerically improving without reaching statistical significance (53.4% versus placebo 44.2%; OR: 1.45; 95% CI: 0.80–2.63), the 3-year survival outcomes were significantly improved with the addition of durvalumab (3-year invasive disease-free survival [iDFS]: 86% versus 77%; HR: 0.48; 95% CI: 0.24–0.97; 3-year OS: 95% versus 84%; HR: 0.24; 95% CI: 0.08–0.72).32
Adjuvant Therapy for Patients Not Achieving Pathologic Complete Response
Considering that not achieving pCR is associated with higher relapse rates and mortality, clinical trials have evaluated whether additional systemic therapy after surgery can improve long-term outcomes. In patients with TNBC who had residual disease after NAC, adjuvant capecitabine (studied in the CREATE-X trial) resulted in absolute improvements of 14% and 9% in disease free survival (DFS) and OS, respectively, compared to no further systemic therapy.25 In pathogenic germline BRCA1 or BRCA2 mutation carriers with HER2-negative breast cancer who were not achieving pCR after NAC, adjuvant olaparib (studied in the OlympiA trial) resulted in absolute improvements of 9%, 8%, and 4% in iDFS, distant disease free survival (DDFS), and OS, respectively, compared to no further systemic therapy.33 It is important to note that these trials were conducted prior to the results of KEYNOTE-522 being reported and prior to the routine use of perioperative pembrolizumab. As such, patients in the CREATE-X and OlympiA trials did not receive concurrent pembrolizumab. Similarly, patients treated in KEYNOTE-522 did not receive adjuvant capecitabine or olaparib, and were treated with pembrolizumab, irrespective of achievement of pCR or not.24 While concurrent use was not evaluated in any of these studies, the use of capecitabine (for gBCRA wild-type) or olaparib (for gBRCA mutant) along with pembrolizumab in the adjuvant setting for patients with residual disease after receiving NAC is favored. Several studies have previously shown the safety of these combinations.34-36 An ongoing Phase II trial, the MIRANE study (NCT03756298), is evaluating adjuvant atezolizumab plus capecitabine versus capecitabine alone in patients with residual disease after NAC. In carriers of a BRCA mutation who have residual disease after NAC, the use of either olaparib or capecitabine is reasonable. However, olaparib is favored over capecitabine due to its mechanism of action specifically targeting homologous recombination defects present in BRCA-deficient tumors, as well as its superiority over chemotherapy in the metastatic setting.37
Chemo-immunotherapy Related Toxicities
An important consideration regarding the broad adoption of immunotherapy in patients with curable TNBC is the potential development of immune-related adverse events (irAEs). While most irAEs are mild, severe or lifelong irAEs can occur rarely. In KEYNOTE-522, the rate of irAEs in the pembrolizumab arm was 35% compared to 13% in the placebo arm.22 Most of the events were treatable endocrinopathies, such as hypothyroidism (15%) or thyroiditis (2%). However, irAEs with the potential to cause a detriment to quality of life and long-term implications also occurred, including severe skin reactions (6%), hyperthyroidism (5%), gastritis (3%), adrenal insufficiency (3%), pneumonitis (2%), and hypophysitis (2%). The rate of Grade 3 irAEs with pembrolizumab was 13%, with about 11% of events leading to drug discontinuation (compared to 2.6% discontinuation in the control arm). There were also rare, but important, Grade 5 irAEs, including one case of autoimmune encephalitis and one case of fatal pulmonary embolism in the pembrolizumab arm.22 While most of the irAEs in KEYNOTE-522 occurred during the neoadjuvant phase of treatment, irAEs can occur several months or even years after treatment, including after immunotherapy has been discontinued.38 In addition to the more common irAEs reported in the KEYNOTE-522 trial, it is important to note that irAEs can affect any organ. This includes rare cases of colitis, hepatitis, myocarditis, and neurologic syndromes, among others. The management of these irAEs generally includes holding or permanently discontinuing immunotherapy for severe toxicities, and immunosuppression with steroids. However, more severe or steroid refractory toxicities may require escalation of immunosuppression, with their own underlying risks.39 The detailed evaluation and management of these important toxicities are beyond the scope of this review, but there are expert consensus guidelines available from both the European Society of Medical Oncology (ESMO)39 and the American Society of Clinical Oncology (ASCO).38
Given the potential life-long implications of irAEs, an area of unmet need is the identification of patients with early-stage TNBC who are most likely to benefit (or not) from the addition of immunotherapy. Currently, the indication for immunotherapy is exclusively based on tumor size and nodal stage. To date, no other predictive biomarkers have been identified. Promising emerging biomarkers are discussed later in this article. In patients who are at high-risk of developing complications with immunotherapy use (such as those with a history of severe autoimmune disease), chemotherapy alone is utilized. In these cases, dose dense anthracycline and cyclophosphamide (ddAC), followed or preceded by paclitaxel with or without carboplatin, is generally recommended.40,41
CURRENT CONTROVERSIES IN EARLY TRIPLE-NEGATIVE BREAST CANCER
Use of Adjuvant Immunotherapy in Those Who Did Not Receive It in the Neoadjuvant Setting
For patients treated with upfront surgery for clinical Stage I TNBC, and are upstaged to Stage II–III on surgical pathology, the potential utility of administering immunotherapy in addition to chemotherapy in the adjuvant setting remains unknown. In the ALEXANDRA/Impassion 030 trial, patients with resected Stage II and III breast cancer were randomized to adjuvant AC-T alone or AC-T plus 1 year of atezolizumab, and ultimately, there was no difference in DFS in the overall population or in the PD-L1 positive subgroup.42 Similarly, in the A-BRAVE trial, which included patients with residual disease after NAC, or with Stage IIB resected TNBC who had completed all chemotherapy, the use of 1 year of adjuvant avelumab versus observation showed a trend towards improved DFS (the primary endpoint) in the avelumab arm, but without achieving statistical significance. Interestingly, OS (a secondary endpoint) was improved.43 The reason for lack of improvement in DFS while OS improved remains unclear. However, an exploratory unplanned analysis of DDFS also showed an improvement with avelumab, suggesting that the lack of DFS improvement may have been due to non-distant events. Given that about 10% of patients in this trial harbored germline BRCA mutations, it is possible that second primaries or non-breast cancer events may have confounded the results. Full results of the trial, including a breakdown of the types of events, are awaited. The ongoing SWOG 1418 trial (NCT02954874) is evaluating the use of adjuvant pembrolizumab in patients with residual disease after NAC without immunotherapy. In the meantime, the use of pembrolizumab in patients who would have met KEYNOTE-522 criteria based on burden of disease, but did not receive it at the neoadjuvant stage, remains controversial. Nevertheless, it is a reasonable consideration in the authors’ opinions, particularly given the now clear OS improvements seen in long-term follow-up of KEYNOTE-522.23
Estrogen Receptor-Low Breast Cancer
ER-low breast cancer is clinically defined as an ER immunohistochemistry (IHC) expression of 1–10% (based on 2020 ASCO/College of American Pathologists [CAP] guidelines.44 In KEYNOTE-522, TNBC was defined as an ER IHC expression of <1% and a PR expression of <1%. However, several datasets suggest that tumors with ER/PR expression of 1–10% have a similar clinical behavior to TNBC, raising the possibility that immunotherapy may also be beneficial to these patients.45,46 Recent clinical trials have evaluated the use of neoadjuvant immunotherapy in early-stage ER-positive breast cancer, and have shown that the highest pCR rates were seen in those with ER-low breast cancer (ER IHC: 1–10%).47-49 As such, while KEYNOTE-522 excluded these patients, in clinical practice we advocate for the incorporation of immunotherapy in patients with ER-low HER2-negative breast cancer.
Furthermore, patients with ER-low breast cancer may still benefit from adjuvant endocrine therapy. In a recent analysis of over 7,000 patients with Stage I–III ER-low breast cancer receiving chemotherapy, omission of endocrine therapy was associated with significantly worse OS, particularly in patients with residual disease after NAC, as well as those with higher ER levels (6–10%).50
Anthracycline Use in Early triple-negative Breast Cancer
While KEYNOTE-522 was a pivotal study, and is the current standard of care for most women with stage II–III TNBC, the toxicities of a 5-drug chemo-immunotherapy regimen are not trivial. This has resulted in an increased interest in identifying patients who may not require a full, intensive regimen, with renewed interest in potentially avoiding the anthracyclines in patients who can safely omit them. An anthracycline-free regimen was studied in the NeoSTOP trial, which randomized patients with Stage I–III TNBC to either 4 cycles of carboplatin and paclitaxel followed by four cycles of doxorubicin and cyclophosphamide, or 6 cycles of carboplatin and docetaxel. In this small trial (N=100), identical pCR rates (54%) were seen in both arms. EFS and OS at 38-month follow-up were also similar, with a more favorable toxicity profile in the non-anthracycline regimen.51 Given the adoption of immunotherapy based on KEYNOTE-522, the single arm NeoPACT trial subsequently evaluated 6 cycles of neoadjuvant carboplatin, docetaxel, and pembrolizumab, which was associated with a pCR rate of 58%, and a 3-year EFS rate of 98% in the group that achieved pCR. This was comparable to the outcomes observed in KEYNOTE-522.52 Notably, in NeoPACT, for patients with node-negative breast cancer, use of this anthracycline-free regimen yielded a pCR rate of 65%, and in immune enriched/high TIL TNBC, 76% achieved pCR. Based on these data, the SCARLET trial is evaluating the non-inferiority of the anthracycline-free chemo-immunotherapy regimen (similar to the NeoPACT regimen) compared to the KEYNOTE-522 regimen in early TNBC (NCT05929768). At present, these data support the potential safe omission of anthracyclines in patients who have a contraindication to the use of anthracycline.
Continuation of Adjuvant Immunotherapy in Patients Achieving Pathologic Complete Response
In KEYNOTE-522, patients continued adjuvant pembrolizumab regardless of whether they achieved pCR or not after completing the neoadjuvant portion of treatment. Prior to KEYNOTE-522, patients achieving pCR were not recommended additional systemic therapy following surgery. As such, another area of debate amongst practitioners is whether pembrolizumab needs to be continued, or if it can be safely omitted in the adjuvant setting for patients achieving pCR. A pooled analysis of 12 trials evaluating NAC (without pembrolizumab) in TNBC found that, in patients who achieved pCR, survival outcomes were very favorable without additional adjuvant therapy.4 In an exploratory subgroup analysis of KEYNOTE-522, patients who achieved pCR in the pembrolizumab arm had numerically superior EFS rates compared to placebo, but this did not reach statistical significance (5-year EFS: 92% versus 88%; HR: 0.65; 95% CI: 0.39–1.08). Importantly, the 5-year OS was nearly identical in the pembrolizumab and placebo arms (95% versus 94%; HR: 0.69; 95% CI: 0.38–1.25).24 Furthermore, in the GeparNuevo trial (which used durvalumab exclusively during the neoadjuvant phase), iDFS, DDFS, and OS were improved compared to chemotherapy alone, suggesting that most of the benefit of immunotherapy may be derived from the neoadjuvant portion.53 The current standard of care recommendation is to continue pembrolizumab regardless of pathologic response; however, the omission of adjuvant pembrolizumab in patients achieving pCR is being prospectively evaluated in the OptimICE-pCR Phase III trial (NCT05812807).
NOVEL THERAPEUTIC STRATEGIES FOR EARLY TRIPLE-NEGATIVE BREAST CANCER
Patients with residual disease after NAC (particularly those with RCB-3) remain at high risk of recurrence and mortality, despite the improvements seen with the addition of immunotherapy in KEYNOTE-522. This is highlighted by an exploratory analysis of the KEYNOTE-522 trial, where patients achieving RCB-2 exhibited 5-year OS rates of 78% and 63% (with and without pembrolizumab, respectively). Meanwhile, those with RCB-3 exhibited similar 5-year OS rates of around 38%, regardless of the receipt of immunotherapy.26 These poor outcomes highlight the need for more effective therapies for these populations.
Strategies being explored for patients with residual disease after NAC include the use of antibody drug conjugates (ADC) that have demonstrated superiority to chemotherapy in the metastatic setting. Examples of trials exploring this strategy include: Tropion-Breast 03 (NCT05629585), which is comparing adjuvant Datopotamab-Deruxtecan (Dato-DXd; AstraZeneca, Cambridge, UK, and Daiichi Sankyo, Chuo City, Japan) with or without durvalumab versus treatment of physician’s choice; and the ASCENT-05/OptimICE-RD (NCT05633654) trial, which is evaluating adjuvant sacituzumab govitecan plus pembrolizumab versus treatment of physician’s choice. Table 1 displays selected ongoing clinical trials in early-stage TNBC.
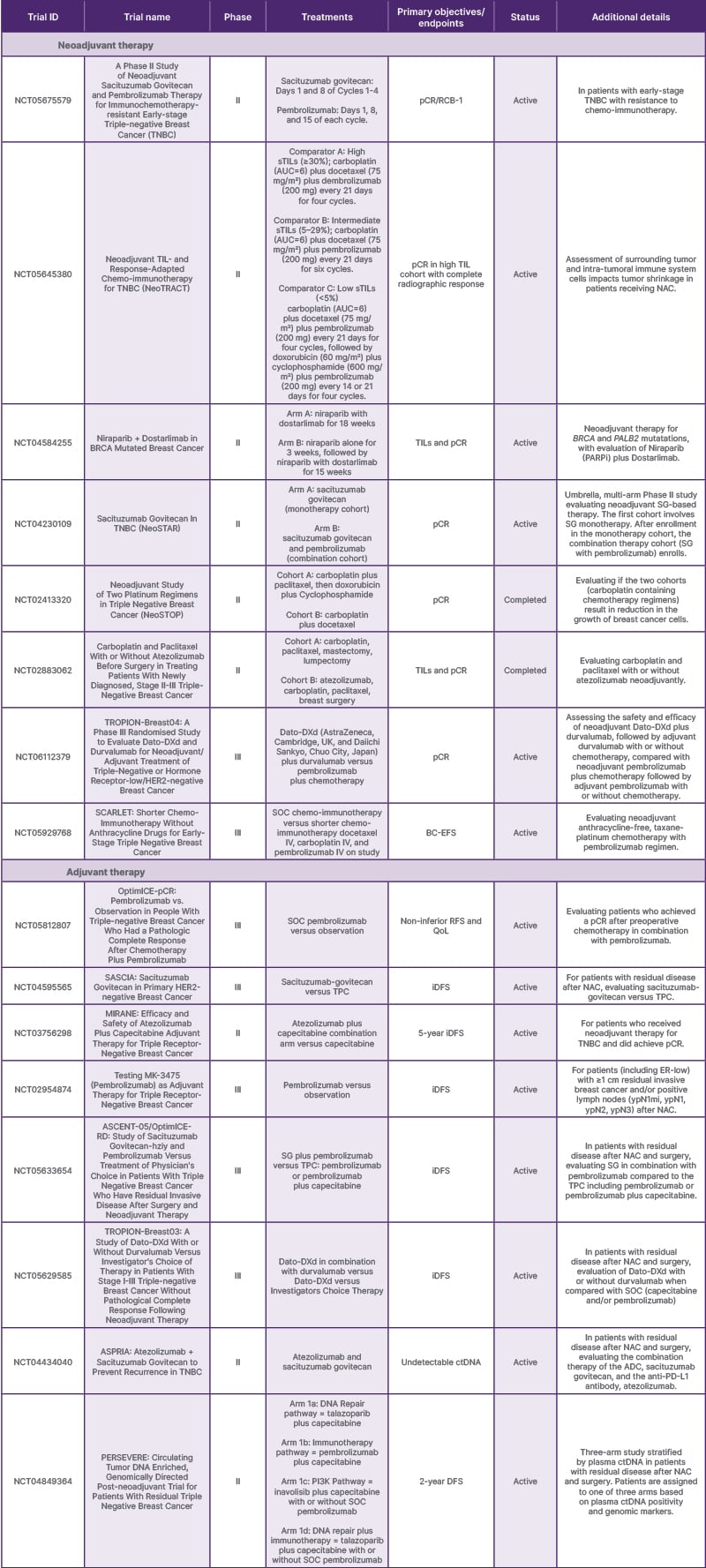
Table 1: Select ongoing or recently completed neoadjuvant and adjuvant interventional trials in early triple-negative breast cancer in the United States in 2025.
Select trials from clinicaltrials.gov.54
ADC: antibody drug conjugate; AUC: area under the curve; BC-EFS: breast cancer event-free survival; ctDNA: circulating tumor DNA; Dato-DXd: Datopotamab-Deruxtecan; DFS: disease free survival; ER: estrogen receptor; iDFS; invasive disease free survival; NAC: neoadjuvant chemotherapy; PARPi: poly (ADP-ribose) polymerase inhibitors; pCR: pathologic complete response; PD-L1: programmed death-ligand 1; QoL: quality of life; RCB: residual cancer burden; RFS: recurrence-free survival; SG: sacituzumab govitecan-hziy; SOC: standard of care;TIL: tumor infiltrating lymphocyte; TNBC: triple-negative breast cancer; TPC: treatment of physician’s choice.
Given the success of ADCs in the metastatic setting, trials are also evaluating whether ADCs could be used during the neoadjuvant treatment phase instead of (or in addition to) chemo-immunotherapy. In the platform I-SPY2 trial, Dato-DXd was evaluated in combination with durvalumab for 12 weeks as a neoadjuvant therapy for patients with HER2-negative breast cancer. Patients with a high likelihood of achieving pCR (evaluated via complete response on MRI and a negative biopsy in the tumor bed area) were allowed to proceed to surgery without additional chemotherapy. A total of 33% of patients were able to skip traditional chemotherapy and go to surgery after 4 cycles of Dato-DXd plus durvalumab. The TNBC subtype had a modeled pCR rate of 44%, and in those with an immune positive response predictive subtype, the modeled pCR rate was 65%.55 Neoadjuvant Dato-DXd plus durvalumab is being further evaluated in early-stage TNBC as part of the ongoing TROPION-Breast04 trial (NCT06112379), and will be compared against the KEYNOTE-522 regimen.
FUTURE DIRECTIONS
Use of Biomarkers to Tailor Treatment Strategies in the Early-Stage Setting
A number of biomarkers (e.g., tumor-infiltrating lymphocytes [TIL], PD-L1, tumor mutational burden, TNBC subtype, homologous recombination deficiency [HRD], and gene expression signatures) have been shown to be highly associated with pCR after NAC, as well as with clinical outcomes in patients receiving and not receiving chemotherapy. In the future, these and other biomarkers may help identify patients who need more or less intensive systemic therapy, including the potential identification of patients at the lowest risk who may safely omit systemic therapy altogether. However, as of this review, none of these biomarkers have been able to identify patients who benefit or do not benefit from the addition of immunotherapy.
PD-L1
PD-L1 can be expressed on tumor receptor surfaces or surrounding tumor-infiltrating immune cells, which play an important role in antitumor immune reactions. PD-L1 expression is noted in approximately 40–65% of TNBC tumors.56,57 In the metastatic setting, PD-L1 expression has been shown to identify patients most likely to benefit from the addition of immunotherapy.27 However, in several studies evaluating PD-L1 as a biomarker in the early-stage setting (including KEYNOTE-522), immune checkpoint inhibitor benefit did not correlate with PD-L1 expression.23 Levels of PD-L1 expression were prognostic, with higher levels being associated with higher pCR rates in both the immunotherapy and chemotherapy alone arms.23
Tumor-infiltrating lymphocytes
Multiple studies have shown that higher levels of TILs are associated with improved outcomes in patients with TNBC in multiple settings. Among patients with early TNBC treated with systemic therapy, higher TILs are associated with improved survival after adjuvant chemotherapy, and a higher rate of pCR with neoadjuvant therapy.58,59 In a large retrospective cohort study evaluating patients with TNBC treated with locoregional therapy only (not receiving neoadjuvant or adjuvant chemotherapy), higher TILs were associated with improved iDFS, DDFS, and OS. Notably, patients with Stage I TNBC and TILs ≥50% showed 5-year recurrence free survival, distant relapse free survival, and OS that approached or exceeded 90%.60 Ongoing studies, such as EORTC 2275/OPTImaL (NCT06476119) and ETNA (NCT06078384), are prospectively evaluating whether high levels of TILs can be used as a biomarker for identifying patients with Stage I TNBC who may safely omit chemotherapy or be treated with immunotherapy alone.
BRCA and PALB2 alterations
Germline BRCA mutations have been found in 10–20% of TNBC, and somatic mutations have been found in 3–5%.61 Germline BRCA and PALB2, along with somatic BRCA, have been found to confer sensitivity to poly (ADP-ribose) polymerase inhibitors (PARPi). Furthermore, platinum agents show increased efficacy in this population. BRCA1 and BRCA2 are tumor suppressor genes, and they encode proteins that assist in the repair of double-strand DNA breaks via the homologous recombination pathway.62 In the metastatic setting, and in the adjuvant setting, PARPis are approved as standard therapies for patients with germline BRCA mutations.61,62 A number of studies have evaluated the activity of PARPi in the neoadjuvant setting. In the NEOTALA trial, neoadjuvant talazoparib (as a single agent) in patients with operable gBRCA-mutated TNBC was associated with a pCR rate of 53%. However, 16% of patients progressed through this regimen, which is higher than the rate of progression observed with most neoadjuvant chemotherapy regimens.63 Given potential synergy, the neoadjuvant PARPi, niraparib, is currently being evaluated in combination with the immunotherapy, dostarlimab, in the TBCRC056 clinical trial (NCT04584255). If successful, this strategy may offer a promising chemotherapy-free neoadjuvant treatment option for these patients in the future.64 Figure 2 highlights various ongoing targets in the neoadjuvant setting in TNBC.
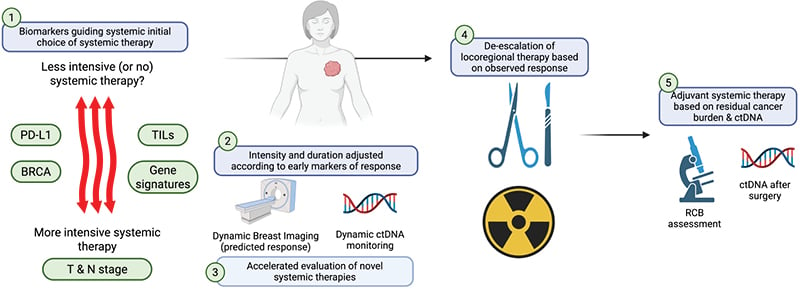
Figure 2: The neoadjuvant systemic therapy platform in triple-negative breast cancer: opportunities for truly personalized therapy.
Created with BioRender® (Toronto, Canada).
ctDNA: circulating tumor DNA; N: nodal status; PD-L1: programmed death-ligand 1; RCB: residual cancer burden;
T: tumor size; TIL: tumor infiltrating lymphocyte.
Homologous recombination deficiency
Approximately 60% of patients with TNBC have HRD.14 This is an impairment of homologous recombination, and cells with this feature utilize non-homologous end joining, which can lead to genomic damage accumulation for which PARPi can be effective. While BRCA genes comprise the homologous recombination repair pathway, there are also other homologous recombination genes that can be compromised. For those, PARPi and platinums may be effective.65
Gene expression profiles
Gene expression signatures are also being explored in TNBC.66 One such assay is TNBC-DX (REVEAL GENOMICS®, Barcelona, Spain) which incorporates a 10-gene signature with a 4-gene proliferation signature, along with tumor and nodal status staging information. This assay, developed using data from nearly 1,300 patients with early TNBC from three clinical trials, reports two scores: the TNBC-DX pCR score (from 0–100), and the TNBC-DX risk score (from 0–100). These scores are associated with the likelihood of achieving pCR after neoadjuvant therapy, and with long term survival outcomes, respectively.67 Before routine clinical use, this assay requires further validation, and importantly, it has not been evaluated in patients with early-stage TNBC treated with locoregional therapy only. Due to this, it is unclear whether TNBC-DX can identify patients for whom systemic therapy can be safely omitted.
Other potential biomarkers
An exploratory biomarker analysis of KEYNOTE-522 evaluated multiple factors potentially associated with pCR and EFS. In this analysis, tumor mutational burden was positively associated with higher pCR in both arms, but with EFS, only in the pembrolizumab arm. A T cell-inflamed gene expression profile was positively associated with pCR and EFS in both arms (with and without pembrolizumab).68 Secondary analysis showed that BRCA and HRD status, as well as PTEN gene loss, were associated with pCR in both groups.68
Refining Recurrence Risk with Minimal Residual Disease Assessment
Current recurrence risk assessment is almost exclusively based on tissue-based response to neoadjuvant therapy (i.e., assessment of RCB) and on clinical stage. Given the emergence of circulating tumor DNA (ctDNA) assays with increasing sensitivity and specificity, there is interest in evaluating whether blood-based monitoring of minimal residual disease could further refine risk assessment. The presence of positive ctDNA has been found to be associated with higher risk of relapse in early-stage TNBC.69-71 Several ongoing trials are evaluating treatment strategies aimed at the treatment of positive ctDNA in an effort to prevent or delay relapse.69 The ASPRIA trial (NCT04434040) is evaluating the use of adjuvant sacituzumab govitecan plus atezolizumab in patients with residual disease after NAC who are found to have positive ctDNA, and is testing whether treatment leads to ctDNA clearance at 18 weeks. The PERSEVERE trial (NCT04849364) is evaluating the potential efficacy of genomically-directed therapy (e.g., PI3K inhibition, PARPi, immunotherapy) in patients with residual disease after NAC and positive ctDNA with specific genomic targets. At present, intervention using treatment based on the detection of minimal residual disease in TNBC has not yet been shown to improve clinical outcomes, and remains experimental.
CONCLUSION
Significant progress has been made in the management of early-stage TNBC, particularly with the shift towards neoadjuvant therapy and the incorporation of immunotherapy. Ongoing key questions in the field include the optimal chemotherapy partner to use with immunotherapy, the need for additional immunotherapy in the context of pCR, and the sequencing of standard adjuvant therapies and use of novel adjuvant therapies (e.g., ADCs) in the context of residual disease. Furthermore, biomarker analyses to better select neoadjuvant and adjuvant treatments (and candidates for the safe omission of systemic therapy) are needed. Ultimately, the primary goal for the treatment of patients in the early-stage setting is to improve overall survival outcomes and decrease the risk of recurrence while minimizing systemic therapy toxicity. It is possible that, in the future, the use of artificial intelligence and machine learning, along with the incorporation of high-risk clinical features and select biomarkers, may enter the scene in order to better predict response and resistance to therapies, and allow for optimal treatment selection.







