Abstract
Fibrolamellar hepatocellular carcinoma (FLC) is a rare liver malignancy typically affecting young adults without underlying liver disease. The case presented here explores the efficacy of combining transarterial chemoembolization (TACE) with immunotherapy and anti-angiogenic therapy.
A 20-year-old woman presented with diffuse abdominal pain, nausea, and vomiting. Imaging revealed a large hepatic mass measuring 181 mm, and multiple pulmonary nodules. Histopathological examination confirmed well-differentiated, unresectable FLC. Systemic treatment with atezolizumab and bevacizumab was considered appropriate; however, delays due to its off-label status in FLC led to the use of TACE as a bridging intervention. Three sessions of TACE were completed prior to the initiation of immunotherapy, which was subsequently administered for 22 cycles.
Although radiological criteria for partial response according to RECIST 1.1 were not met, a 26.7% reduction in tumor size was observed, along with stability of pulmonary lesions. The patient achieved a progression-free survival of 18.13 months and an overall survival of 22.23 months. These findings highlight the potential of a combined locoregional and systemic approach in the management of unresectable FLC, and represent the first reported case of this strategy in Mexico.Further investigation into immunotherapy-based, multimodal regimens is warranted, highlighting the importance of collaborative efforts to improve outcomes in rare hepatic malignancies.
Key Points
1. Fibrolamellar hepatocellular carcinoma (FLC) is a rare, aggressive liver cancer affecting young adults without underlying liver disease. This case addresses the urgent need for innovative therapies, as advanced FLC lacks standardized treatments and carries a poor prognosis, with survival rates below 20% in unresectable cases.
2. Combining transarterial chemoembolization with systemic atezolizumab (anti-PD-L1) and bevacizumab (anti-VEGF) achieved prolonged progression-free survival (18.1 months) and overall survival (22.2 months), suggesting synergistic efficacy through immune modulation, anti-angiogenesis, and locoregional control in advanced FLC.
3. This case highlights the potential of immunotherapy-based combinations in FLC and emphasizes the importance of reporting rare malignancies to build evidence, guide clinical trials, and improve access to novel therapies in resource-limited settings.
BACKGROUND
Liver cancer is a leading global health concern, ranking as the sixth most common cancer and the third deadliest in 2022, with over 866,000 new cases and 758,000 deaths worldwide.1 While hepatocellular carcinoma (HCC) accounts for 90% of liver cancers, fibrolamellar carcinoma (FLC) is a rare, distinct entity, comprising only 1–9% of cases.2,3 Unlike conventional HCC, FLC primarily affects adolescents and young adults without underlying liver disease or cirrhosis, and typically presents with vague, non-specific symptoms such as abdominal pain, nausea, and weight loss.4,5 In Mexico, only seven cases treated at the National Institute of Medical Sciences and Nutrition Salvador Zubirán between 1980–1999 have been documented.6
FLC is characterized by unique clinical, genetic, and pathological features, including normal α-fetoprotein (AFP) levels in most cases and a distinctive histological pattern of lamellar fibrosis surrounding large polygonal tumor cells.7 Imaging commonly reveals a heterogeneous hepatic mass with peripheral calcifications, although definitive diagnosis requires histopathological confirmation.8 Surgical resection remains the only curative option, with an 80% five-year survival rate for localized disease. However, recurrence is common, and advanced, unresectable cases have poor prognoses, with survival rates as low as 10–20%.9,10
At present, treatment options for unresectable FLC remain limited, and no standard of care has been established. While platinum-based chemotherapy regimens are commonly employed, immune checkpoint inhibitors and anti-angiogenic therapies have recently shown promise.11 Nevertheless, little is known about their use in combination with other treatment modalities. In this context, a case is presented of advanced unresectable FLC treated with a novel multimodal strategy involving transarterial chemoembolization (TACE), atezolizumab, and bevacizumab. This report aims to contribute to the limited evidence of this entity and highlight the potential of immunotherapy-based combination regimens in the management of this rare malignancy.
CASE PRESENTATION
In May 2020, a 20-year-old Latin American woman presented with diffuse abdominal pain, nausea, and vomiting. Her medical history included systemic lupus erythematosus, diagnosed in 2018 and is currently inactive, and a recent caesarean section 4 weeks prior. Physical examination revealed tenderness in the right hypochondrium and a palpable hepatic border below the costal margin.
Serological analysis indicated Grade I normochromic anemia (hemoglobin: 10.5 g/dL), elevated GGT (183 U/L), LDH (754.3 U/L), and AFP (22.9 ng/mL), with no other significant findings. A general urine test revealed findings suggestive of a urinary tract infection (positive nitrites, leukocyte esterase 25 U/L, leukocytes 7 per field). The viral profile for hepatitis A, B, and C was negative.
Abdominal ultrasound found a heterogeneous mass measuring 143×118 mm accompanied by multiple peripheral lesions throughout the liver parenchyma. Multiphasic thoraco-abdominal-pelvic CT showed a heterogeneous lesion measuring 181x131x161 mm with a hypodense center and multiple calcifications. In the non-contrast phase, the lesion had a density of 72 Hounsfield units (HU), increasing to 132 HU post-contrast. Additionally, multiple pulmonary nodules ranging from 4×4 mm to 10×9 mm were identified,with a mean density of 38 HU (Figure 1).
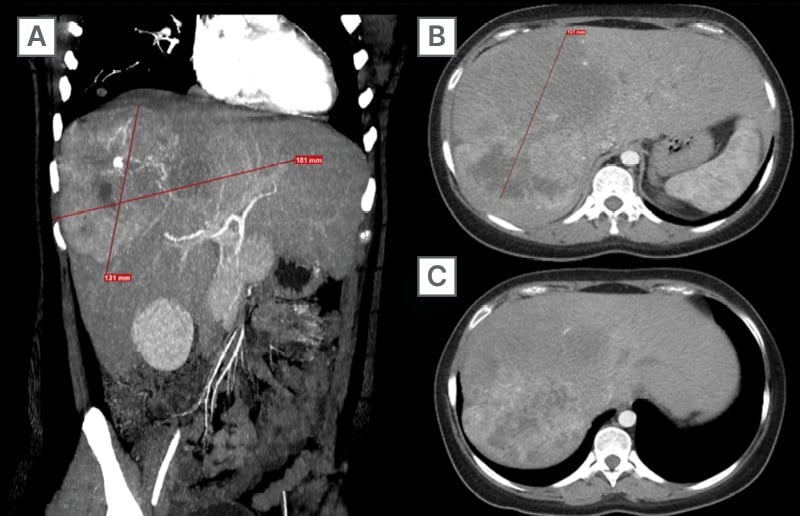
Figure 1: Multiphase staging CT.
A) Coronal slice showing the primary lesion (181×131 mm) with irregular borders and variable density in the contrast-enhanced phase.
B) Axial slice at the T9 level displaying the axial dimension of the lesion (164 mm) with post-contrast enhancement features.
C) Axial slice at the T11 level demonstrating how the lesion becomes peripheral without involving other structures.
Histopathological examination of the liver biopsy confirmed a diagnosis of well-differentiated FLC. The disease was staged as IVB according to the American Joint Committee on Cancer (AJCC) classification, and as stage C under the Barcelona Clinic Liver Cancer (BCLC) system, based on extensive hepatic involvement and distant metastases.
A dual therapeutic approach was implemented, comprising locoregional therapy with TACE administered in three sessions at 3-week intervals starting in June 2020, and systemic therapy with atezolizumab plus bevacizumab, initiated in July 2020 and continued every 21 days until disease progression, completing 22 cycles.
Follow-up imaging in August 2020 showed a 26.7% reduction in the size of the primary hepatic lesion, corresponding to stable disease as per RECIST 1.1 criteria (Figure 2). Disease stability was maintained for over 1 year with periodic imaging assessments. However, progression was observed in November 2021, with increased hepatic tumor size, new metastatic lesions, and clinical deterioration. Due to financial limitations and declining performance status, further lines of therapy were not pursued. The patient was transitioned to palliative care and passed away in March 2022.
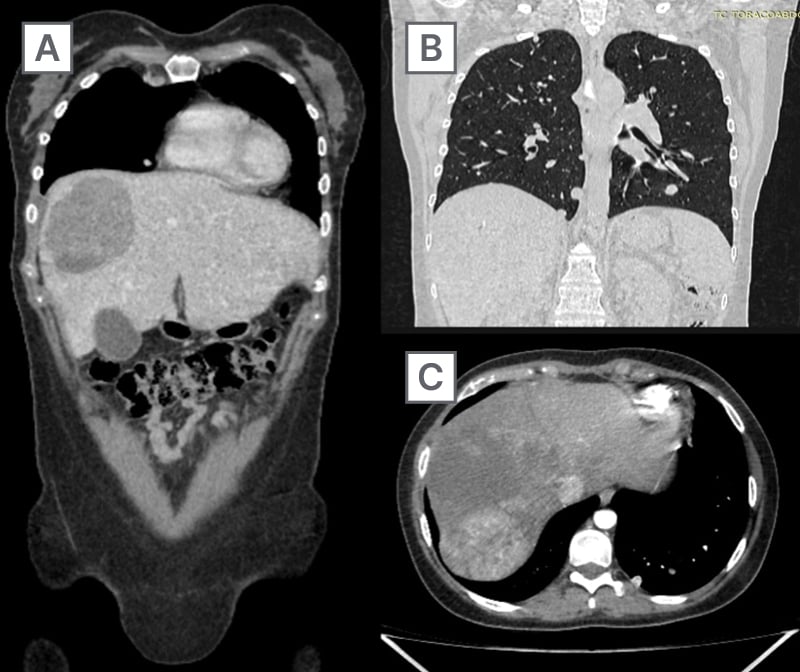
Figure 2: Treatment assessment (December 2020).
A) Significant reduction in lesion size (130 × 110 mm) is observed. The mass shows decreased contrast enhancement, consistent with treatment-induced tumor necrosis.
B) Mediastinal lymph nodes and multiple pulmonary nodules are identified, with no changes in size or number.
C) Necrotic areas and reduced tumor volume are evident after treatment, with less defined borders and decreased contrast uptake.
DISCUSSION
The clinical presentation, serological profile, and radiological findings in this case aligned with what is commonly reported in FLC. The patient’s vague abdominal pain, nausea, and vomiting, in addition to mildly elevated GGT, LDH, and AFP, are typical features of this rare malignancy. No records of the patient’s prenatal follow-up could be obtained; yet, it is noteworthy that no atypical findings were reported during pregnancy, despite the tumor’s considerable size (181 mm), which would be expected to cause abdominal distension. This highlights the diagnostic challenges posed by FLC, particularly in young women.
Following diagnosis, a multidisciplinary team deemed the lesion unresectable, limiting therapeutic options. Although the IMbrave150 trial demonstrated the superiority of atezolizumab plus bevacizumab over sorafenib in unresectable HCC, FLC was excluded from this trial, leaving a significant gap in direct clinical evidence.12 Nonetheless, the hepatic tumor microenvironment and emerging insights into FLC immunobiology provide a theoretical rationale for the use of this combination.
The liver is a highly vascularized organ that also functions as an immunological barrier, continuously exposed to gut-derived antigens via the portal circulation. It maintains a tolerogenic state through the action of Kupffer cells, dendritic cells, and other components of the innate and adaptive immune systems.13,14 Given this, it is not surprising that FLC demonstrates immunosuppressive behavior. This was confirmed by a study conducted at Johns Hopkins Hospital including 25 patients with FLC, where 63% showed high PD-L1 expression (>5%) in tumor cells and 69% in infiltrating immune cells, predominantly at the tumor–stroma interface, suggesting an immune-evasive phenotype.4
Furthermore, hypoxia-driven release of VEGF in hepatic tumors not only promotes angiogenesis and progression, but also contributes to immunosuppression by recruiting regulatory T cells, tumor-associated macrophages, and myeloid-derived suppressor cells. These immune populations secrete inhibitory cytokines such as IL-10 and TGF-β, impairing dendritic cell maturation and CD8+ T cell activity.15 These findings support the rationale for combining VEGF and PD-L1 blockade in selected liver cancers.
Although systemic therapy was considered the most appropriate strategy, the use of atezolizumab plus bevacizumab was classified as off-label in the context of FLC. Therefore, a formal approval process was required through both institutional and pharmaceutical channels, leading to a delay of approximately 1 month. As a result, the interventional radiology department proposed TACE as a bridging intervention.
FLC, like conventional HCC, is a hypervascular tumor and is therefore theoretically susceptible to embolization. Although TACE is not part of the standard of care for FLC, there are isolated reports describing its use in both perioperative and advanced settings with favorable outcomes.16 In this case, three TACE sessions were administered at 3-week intervals. Due to financial and logistical constraints, intermediate radiological assessment between sessions was not feasible, and evaluation was performed only after completing the full course.
There are several limitations in this case that must be acknowledged. Firstly, PD-L1 expression could not be assessed due to tissue unavailability, and genomic testing for the DNAJB1–PRKACA fusion, which is considered a pathognomonic biomarker for FLC, was not performed owing to economic barriers.17 Additionally, the lack of interim radiological monitoring during TACE precluded the ability to determine the independent contribution of each treatment modality. These limitations reflect common challenges in real-world oncology settings and highlight the need for improved access to molecular diagnostics.
Despite not meeting the threshold for partial response (≥30% reduction in size according to RECIST 1.1), the reduction in tumor volume (26.7%) (Table 1), radiological stability of pulmonary nodules, and prolonged progression-free and overall survival (18.13 and 22.23 months, respectively), suggest that a multimodal approach incorporating locoregional and systemic immuno-oncological strategies may be beneficial, even in advanced FLC. To the authors’ knowledge, this is the first case in Mexico to report clinical benefit using this combination in FLC, and it adds to the limited body of literature informing the treatment of this rare malignancy.
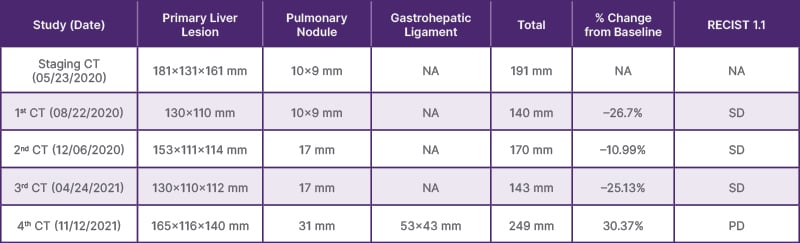
Table 1: Staging and assessing treatment response.
PD: progressive disease; SD: stable disease; NA: not applicable.
CONCLUSION
The treatment of unresectable FLC remains a significant challenge due to its rarity and the lack of standardized therapeutic options. This case illustrates the potential benefit of a multimodal approach combining TACE, immunotherapy, and anti-angiogenic therapy, achieving prolonged progression-free and overall survival in a real-world setting. To the authors’ knowledge, this represents the first reported case in Mexico utilizing this strategy in FLC.
While these results are promising, further research is essential to validate the efficacy of such strategies in larger cohorts. This case underscores the importance of exploring innovative treatment combinations and fostering international collaboration to advance the management of this rare malignancy. Ultimately, improving access to advanced therapies and conducting prospective studies will be critical to better address the needs of patients with FLC.63.







