Author: Helen Boreham1
1. HB Medical (UK) Ltd, Wetherby, UK
Disclosure: Boreham has declared no conflicts of interest.
Disclaimer: Trastuzumab deruxtecan has not been approved by the FDA for the neoadjuvant or adjuvant setting for early-stage, HER2-positive breast cancer. Trastuzumab deruxtecan is currently approved for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received a prior anti-HER2-based regimen either in the metastatic setting, or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within 6 months of completing therapy; and for the treatment of adult patients with unresectable or metastatic hormone receptor-positive, HER2-low, or HER2-ultralow breast cancer that has progressed on one or more endocrine therapies or chemotherapy in the metastatic setting.
Support: The publication of this article was funded by AstraZeneca.
Keywords: Adjuvant, anthracycline-sparing, antibody–drug conjugate (ADC), DESTINY-Breast05, DESTINY-Breast11, early breast cancer (eBC), human epidermal growth factor receptor 2-positive (HER2+), high risk, neoadjuvant, trastuzumab deruxtecan (T-DXd).
Citation: Oncol AMJ. 2026;3[Suppl 1]:2-13. https://doi.org/10.33590/oncolamj/GFDW6100
Meeting Summary
Treatment of early breast cancer (eBC) is continuing to advance, and pivotal Phase III trial data released at the San Antonio Breast Cancer Symposium (SABCS) 2025 signal a potential reshaping of clinical management for patients with human epidermal growth factor receptor 2-positive (HER2+) high-risk disease. In the pivotal DESTINY-Breast05 study, trastuzumab deruxtecan (T-DXd) showed statistically significant and clinically meaningful improvement in invasive disease-free survival (IDFS) versus trastuzumab emtansine (T-DM1) in the post-neoadjuvant residual disease setting. Additional results from the DESTINY-Breast05 interim analysis, presented at SABCS, confirmed that this survival advantage was consistent regardless of patients’ prior neoadjuvant chemotherapy (NACT) or HER2 status. In the DESTINY-Breast 11 study, neoadjuvant treatment with T-DXd followed by paclitaxel, trastuzumab, and pertuzumab (THP) yielded a high pathological complete response rate (pCR) of 67.3% in high-risk HER2+ eBC. The most recent data from this study, presented at SABCS, highlighted T-DXd’s manageable safety profile and improvements in key patient-reported outcomes (PRO) compared with dose-dense doxorubicin plus cyclophosphamide and THP (ddAC-THP). In DESTINY-Breast11, T-DXd demonstrated reduced toxicity compared to ddAC-THP, and was associated with a lower patient-reported treatment burden and improved physical functioning.
Taken together, these findings suggest a potential paradigm shift in the treatment of HER2+ eBC, away from traditional anthracycline-based therapy and towards antibody–drug conjugate (ADC) regimens with improved efficacy and tolerability. In particular, the clinical benefit and favorable safety profile of T-DXd demonstrated in the DESTINY-Breast-05 and 11 studies may support its movement into curative-intent, early-stage treatment spanning both adjuvant and post-neoadjuvant settings.
Background
HER2-directed therapies have dramatically improved outcomes for patients with HER2+ eBC.1 However, up to half of patients experience residual invasive disease after NACT and trastuzumab-based treatment, which is linked to an elevated risk of distant recurrence and death.2,3
The Phase III KATHERINE study signaled a paradigm shift in the treatment of residual invasive HER2+ eBC, establishing T-DM1 as the current standard of care (SOC) in the adjuvant setting. KATHERINE showed that adjuvant treatment with T-DM1 after trastuzumab-based neoadjuvant therapy significantly reduced the risk of invasive disease recurrence by 46% and improved overall survival by 34% compared with trastuzumab alone. However, patients in KATHERINE had a high risk of recurrence if lymph node positive at the time of surgery (7-year IDFS: 72%) or if inoperable at baseline (7-year IDFS: 67%). KATHERINE also showed less benefit from T-DM1 in patients with HER2 immunohistochemistry (IHC) 2+ status as compared to IHC 3+ (7-year IDFS: 72% versus 83%).4-6
Open clinical questions therefore remain, and there is an ongoing need to improve outcomes in high-risk patients with HER2+ eBC. This article provides an up-to-date summary of the most practice-relevant findings on HER2+ eBC presented at SABCS 2025, focusing on data supporting the potential move of ADCs into earlier, curative-intent treatment settings. SABCS itself represents the largest breast cancer (BC) meeting in the world, and brings together leading international experts in clinical, translational, and basic research.
DESTINY-Breast05
In the interim analysis of the Phase III DESTINY-Breast05 study, T-DXd improved the primary endpoint of IDFS compared to T-DM1 in patients with residual invasive HER2+ eBC after neoadjuvant therapy, with a hazard ratio of 0.47 (95% CI: 0.34–0.66; p<0.0001).7 Additional subgroup and safety analyses from the DESTINY-Breast05 trial, aiming to further characterize the benefit-risk profile of post-neoadjuvant T-DXd in patients with HER2+ eBC with residual invasive disease, were presented at SABCS by Sibylle Loibl from the German Breast Group (GBG), Neu-Isenburg; and Goethe University Frankfurt, Germany, and published simultaneously.8,9
DESTINY-Breast05 is an ongoing global, multicenter, randomized, open-label Phase III trial involving patients with residual disease in the breast or axillary lymph nodes after NACT plus HER-2-directed therapy. Eligible patients must have high-risk disease, defined as presentation prior to neoadjuvant therapy with inoperable eBC (cT4, N0-3, M0 or cT1-3, N2-3, M0) or operable eBC (cT1-T3, No-1, M0), with axillary node-positive disease (ypN1-3) after neoadjuvant therapy. Radiotherapy (RT) is permitted in the study at the investigator’s discretion, initiated either alongside systemic therapy (concurrent) or completed first (sequential).8,9
Subgroup analysis of results from DESTINY-Breast05 by prior NACT, presented by Loibl, showed that patients derived a significant survival benefit from T-DXd regardless of whether their neoadjuvant regimen was anthracycline or platinum-based. The hazard ratio for IDFS with T-DXd versus T-DM1 was 0.45 and 0.54 for patients treated with prior anthracyclines or prior platinum-based therapy, respectively (Figure 1A).9
Similar outcomes were also seen in the subgroup analysis by IHC status, where the survival benefit of T-DXd was retained regardless of patients’ IHC classification. Patients with an IHC score of 3+ or HER2 IHC 2+/1+ and amplification on in situ hybridization treated with T-DXd showed superior IDFS to those receiving T-DM1 (Figure 1B).9
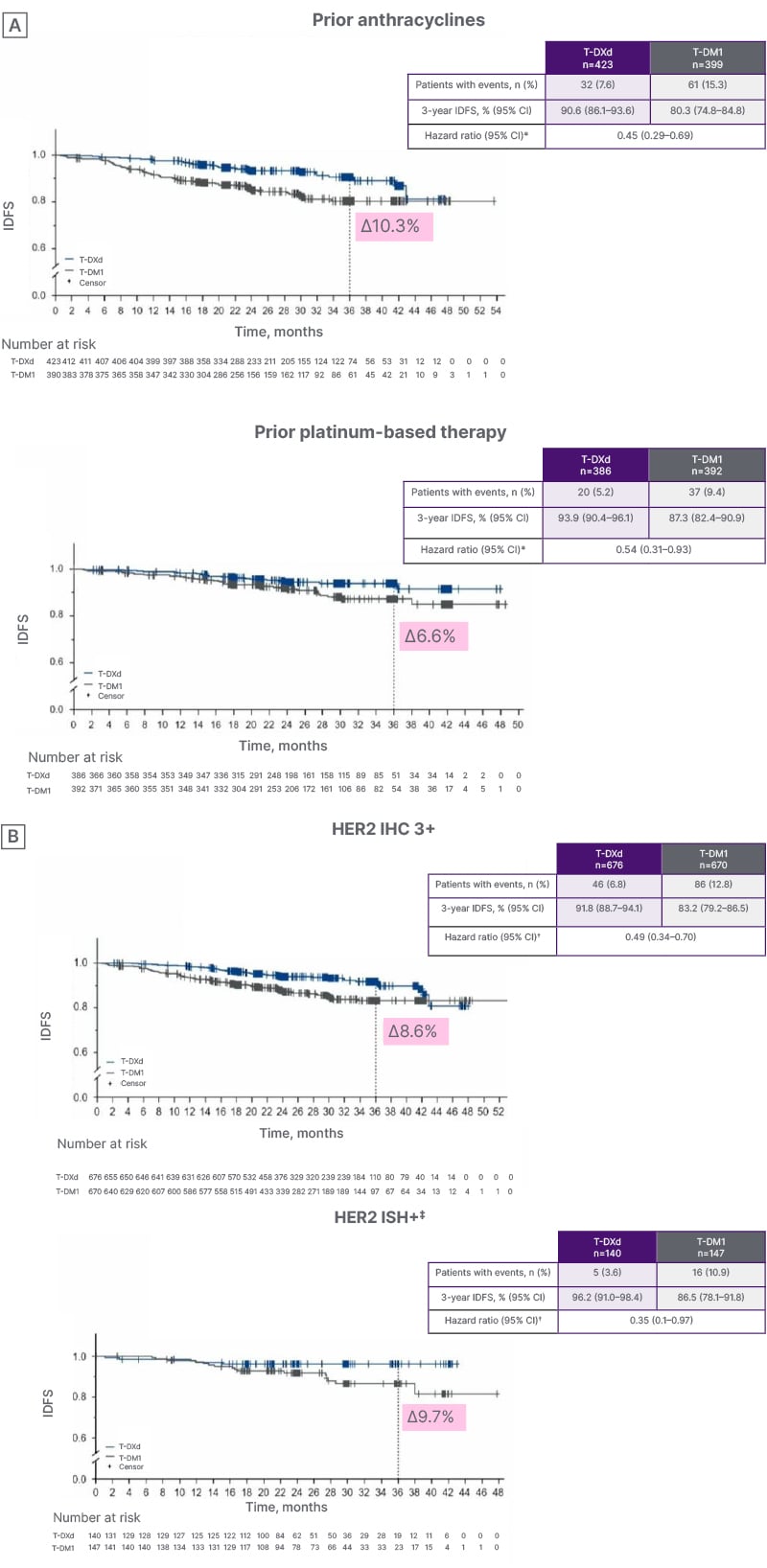
Figure 1: DESTINY-Breast05: invasive disease-free survival subgroup analysis by A) prior neoadjuvant chemotherapy and B) human epidermal growth factor receptor 2 status.9
*By central test from pre-neoadjuvant core sample or surgical specimen.
?Hazard ratio and 95% CI from unstratified Cox proportional hazards model.
‡ISH+ included centrally assessed HER2 IHC 1+ (T-DXd: n=11; T-DM1: n=14) and IHC 2+ (T-DXd: n=129; T-DM1: n=133). Two patients were IHC 2+/ISH- and not included.
HER2: human epidermal growth factor receptor 2; IDFS: invasive disease-free survival; IHC: immunohistochemistry; ISH: in situ hybridization; T-DM1: trastuzumab emtansine; T-DXd: trastuzumab deruxtecan.
Turning to safety findings from DESTINY-Breast05, Loibl outlined the low-dose, non-contrast CT requirements in place for identifying interstitial lung disease (ILD) and radiation pneumonitis (RP) in the study. Patients receiving both sequential and concurrent RT underwent chest CT at baseline and prior to infusion for Cycles 3, 7, and 11, then again at 40 (+7) days follow-up, with additional CT performed at the appearance of any suggestive signs or symptoms. In line with clinical practice, the study protocol also incorporated comprehensive guidelines for managing cases of drug-related ILD and RP.9
The total rate of any-grade adjudicated drug-related ILD in DESTINY-Breast05 was 9.6% in the T-DXd arm compared to 1.6% for T-DM1-treated patients. Notably, the timing of adjuvant RT did not impact either the incidence or severity of drug-related ILD in either arm and, in the T-DXd arm specifically, did not affect the time to onset or duration of this adverse event (AE). The time to onset of ILD was around 120 days in patients receiving T-DXd, irrespective of their adjuvant RT regimen, with an average duration of just over 70 days. Most patients who experienced drug-related ILD in the study had recovered or were in the process of recovering at the data cut-off.9
Considering investigator-reported RP, Loibl noted that all events were Grade ≤2 and that the overall rate was broadly similar in patients treated with T-DXd and T-DM1, at approximately 30%. However, patients who received sequential adjuvant RT experienced higher RP incidences than those who received RT concurrently. In the T-DXd arm, any-grade RP rates were 34.5% for sequential RT versus 29.2% for concurrent RT, compared to 37.4% versus 26.7%, respectively, in the T-DM1 arm. Time to onset and duration of RP were longer in T-DXd-treated patients who received sequential versus concurrent adjuvant RT. Rates of unrecovered/unresolved RP were also higher in patients who received sequential RT prior to T-DXd (54.5%) as compared with T-DM1 (39.6%). As with ILD, most patients with RP events had recovered or were recovering at the time of data cutoff.9
Considering the clinical practice implications of these findings, Loibl reiterated that the timing of adjuvant RT did not impact either the incidence or the severity of T-DXd-related ILD, and most affected patients either recovered or were recovering from this AE by the end of the study. Importantly, most cases of ILD and RP proved generally manageable with protocol-specific guidelines, and she urged all clinicians to become familiar with this guidance when treating patients with eBC using T-DXd. Overall, this further characterization of the clinical benefit and safety profile supports T-DXd as a potential new SOC in the post-neoadjuvant HER2+ eBC residual invasive disease setting, Loibl concluded.9
DESTINY-Breast11
Moving from the adjuvant to the neoadjuvant setting, Giuseppe Curigliano from the University of Milan, Italy, gave a deep dive into safety findings from the pivotal Phase III DESTINY-Breast11 trial comparing T-DXd, either alone or followed by THP, to the SOC ddAC-THP in patients with HER2+ eBC.10
DESTINY-Breast11 was a global, randomized, multicenter, open-label Phase III trial in patients with previously untreated HER2+ eBC who were high risk defined as: ≥cT3 and N0–3 OR cT0–4 and N1–3; inflammatory BC. In primary results disclosed at the European Society for Medical Oncology (ESMO) Congress 2025, T-DXd-THP achieved a statistically significant and clinically meaningful improvement in the primary endpoint of pCR versus ddAC-THP: pCR rate was 67.3% in the T-DXd-THP arm, an improvement of Δ 11.2% compared to ddAC-THP. The safety profile of T-DXd-THP in the DESTINY-Breast11 trial was also favorable compared with ddAC-THP, with no new safety signals identified. Rates of Grade ≥3 AEs, serious AEs, and AEs leading to treatment interruption were all consistently lower in the T-DXd-THP and T-DXd arms as compared to ddAC-THP.11,12
These additional safety analyses presented at SABCS focused on AEs of special interest, namely ILD/pneumonitis and left ventricular (LV) dysfunction, and clinically relevant AEs such as nausea, vomiting, neutropenia, and peripheral neuropathy. As Curigliano outlined, rates of all-grade adjudicated drug-related ILD/pneumonitis were low and similar across all arms in the study: T-DXd-THP 4.4%, ddAC-THP 5.1%, and T-DXd 4.9% (Figure 2). However, Grade ≥3 events occurred most frequently with ddAC-THP (1.9%) compared with T-DXd-THP (0.6%) and T-DXd alone (0%). Rates of ILD/pneumonitis remained stable with T-DXd-THP, but were higher with ddAC-THP in the THP treatment phase (Cycles 5–8) as compared to Cycles 1–4. The incidence of treatment discontinuations and interruptions due to ILD/pneumonitis was low across treatment arms (between 2–3%), with no dose reductions. Serious ILD/pneumonitis events occurred more often in the ddAC-THP arm (2.9%) than with T-DXd-THP (0.6%) or T-DXd (0.4%). Most patients with Grade ≥2 ILD/pneumonitis events underwent treatment with steroids, and the majority of cases were resolved or resolving by data cut-off (by adjudication, recovered, or resolving at data cutoff: 71% with T-DXd-THP, 88% with ddAC-THP, and 64% with T-DXd.) The median duration of the first event was similar for the T-DXd-THP and ddAC-THP combination regimens (45 and 43 days, respectively), but longer for T-DXd alone (60 days).10
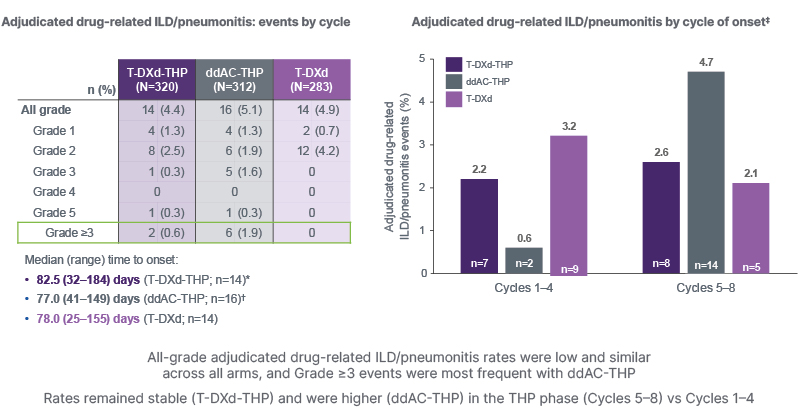
Figure 2: DESTINY-Breast11: adjudicated drug-related interstitial lung disease/pneumonitis events by cycle.10
*Q3W schedule; ?Q2W schedule; ‡percentages are calculated using the number of patients at risk at any point in the cycle window as the denominator, and patients may be counted twice if they had multiple events in different cycles.
Safety analyses included all patients who received at least one dose of any study treatment.
ddAC-THP: dose-dense doxorubicin plus cyclophosphamide followed by paclitaxel, trastuzumab, and pertuzumab; ILD: interstitial lung disease; Q2W: every 2 weeks; Q3W: every 3 weeks; T-DXd: trastuzumab deruxtecan; T-DXd-THP: trastuzumab deruxtecan followed by paclitaxel, trastuzumab, and pertuzumab; vs: versus.
In terms of cardiac safety, Curigliano explained that, due to the anthracycline-containing component, rates of all-grade and Grade ≥3 LV dysfunction were higher in the ddAC-THP arm as compared to the T-DXd-based treatment groups. LV dysfunction occurred in 1.3% and 0.7% of patients in the T-DXd-THP and T-DXd arms, respectively, compared with 6.1% treated with ddAC-THP (respective rates of Grade ≥3 events were 0.3%, 0%, and 1.9%). There were no cardiac failure events in the T-DXd study arms versus four (1.3%) with SOC ddAC-THP.10
Among clinically relevant AEs, nausea and vomiting events were generally low grade across all treatment groups in the study and decreased substantially after Cycles 1–4. Rates of all-grade nausea and vomiting were higher in T-DXd-containing arms than with ddAC-THP, with all-grade nausea occurring in 64.7% and 68.2% of patients in the T-DXd-THP and T-DXd arms, respectively, compared to 51.6% on ddAC-THP. Respective rates of all-grade (≥3) vomiting were 28.8% (0.9%), 31.1% (1.1%), and 21.2% (0.6%). However, use of ≥3 antiemetics was also substantially higher in the ddAC-THP arm, where over half of patients received antiemetics on or prior to Cycle 1 Day 1, compared to only around a third in the T-DXd arms. Curigliano therefore stressed the importance of following guidelines and recommendations for antiemetic use, and reinforced the need for appropriate premedication when treating patients with T-DXd in clinical practice in order to mitigate AEs.10
The overall incidence of hematologic toxicities was lower in the T-DXd-THP treatment arms than in the SOC arm. Specifically, rates of all-grade neutropenia were lower with T-DXd-THP (29.1%) and T-DXd (21.6%) compared to ddAC-THP (44.2%). Granulocyte colony-stimulating factor (GCSF) use was also substantially higher in the ddAC-THP versus the T-DXd treatment groups. Overall rates of peripheral neuropathy were increased with T-DXd-THP (45.0%) as compared to T-DXd (6.7%) and ddAC-THP (35.9%). However, these events were non-serious and generally low grade, with most occurring during the THP treatment phase (Cycles 5–8).10
“These DESTINY-Breast11 safety results support T-DXd-THP as a potential neoadjuvant treatment option for patients with high-risk HER2+ eBC,” Curigliano concluded.
DESTINY-Breast11: Patient-Reported Outcomes
PROs from DESTINY-Breast11 were presented at SABCS by Shanu Modi from the Memorial Sloan Kettering Cancer Center in New York, USA.13 Two secondary endpoints evaluated patient experience during this trial, focusing on physical function and treatment tolerability. Physical functioning was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) physical function scale. Treatment tolerability was evaluated in two ways: a global assessment of side effect bother recorded on the Patient Global Impression of Treatment Tolerability (PGI-TT) instrument and a measurement of 20 individual symptomatic AEs selected from the EORTC and the PRO version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE®; National Cancer Institute, USA) libraries. Six of these symptoms were also evaluated for their impact on functioning during daily activities. All PROs were recorded at baseline and weekly during neoadjuvant therapy, as well as at the end of treatment.13
Results from DESTINY-Breast11 showed that the proportion of patients with maintained or improved physical function on the EORTC QLQ-C30 scale during neoadjuvant treatment was higher in the T-DXd-THP arm than in the anthracycline-containing control arm for the majority of timepoints. Similar findings were observed in the T-DXd monotherapy arm versus ddAC-THP (Figure 3).13
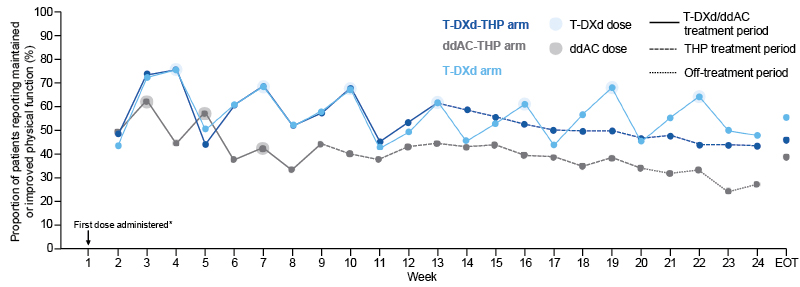
Figure 3: DESTINY-Breast11: physical function change from baseline.13
*The first dose was administered at the start of Week 1. Baseline physical functioning was recorded prior to the first dose of study treatment. Change in physical function was calculated relative to baseline, hence the baseline datapoints are not shown.
Overall compliance rates for the EORTC QLQ-C30: T-DXd-THP, 69.9%; ddAC-THP, 62.6%; T-DXd, 63.8%. Overall compliance rates for the EORTC-IL 19: T-DXd-THP, 74.1%; ddAC-THP, 72.5%; T-DXd, 72.2%. Improvement in physical functioning was defined as an increase of ≥10 points from baseline, deterioration was defined as a decrease of ≥10 points from baseline, and maintained was defined as no improvement or deterioration.
ddAC: dose-dense doxorubicin plus cyclophosphamide; ddAC-THP: dose-dense doxorubicin plus cyclophosphamide followed by paclitaxel, trastuzumab, and pertuzumab; EORTC-IL: European Organisation for Research and Treatment of Cancer Item Library; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality
of Life Questionnaire Core 30; EOT: end of treatment; THP: paclitaxel, trastuzumab, and pertuzumab; T-DXd:
trastuzumab deruxtecan; T-DXd-THP: trastuzumab deruxtecan followed by paclitaxel, trastuzumab, and pertuzumab.
Patient-reported tolerability as measured by PGI-TT was also better in the T-DXd-THP and T-DXd arms compared with ddAC-THP for the majority of timepoints. A higher proportion of patients said that they were “not at all bothered” by the side effects of cancer treatment in the two T-DXd-THP arms versus the control. Conversely, the proportion of patients reporting “quite a lot” or “very much” bother from treatment side effects was greater in the ddAC-THP arm as compared to the two T-DXd treatment groups.13
Of the 20 symptomatic AEs measured for their severity, frequency, or occurrence, the majority were better in the T-DXd-containing arms compared to ddAC-THP. Specifically, appetite loss, chest pain, cough, dyspnea, diarrhea, hot flashes, insomnia, joint pain, mouth/throat sores, muscle pain, numbness/tingling, rash, and taste changes were generally better with T-DXd-THP versus ddAC-THP. On the other hand, fatigue and constipation were judged as being similar. Five symptoms were considered worse with T-DXd-THP versus control: hair loss, nausea, vomiting, headache, and nosebleeds. Broadly similar findings for symptomatic AEs were seen in the comparison of T-DXd alone versus ddAC-THP. Across all study arms, for the six AEs for which interference was measured (insomnia, joint pain, mouth/throat sores, muscle pain, numbness/tingling), there was limited impact on patients’ usual or daily activities from these symptoms.13
In summary, PRO results from the DESTINY-Breast11 study showed that a greater proportion of patients in the T-DXd-containing arms maintained or improved their physical function during neoadjuvant treatment compared with ddAC-THP. Both T-DXd arms also benefitted from a lower patient-reported treatment burden than ddAC-THP, with better overall tolerability, lower symptom bother, and fewer or less severe symptomatic AEs. Interference with daily activities was limited and similar across all three study arms. These PRO results, combined with the favorable efficacy and safety profile of T-DXd-THP versus ddAC-THP, support T-DXd-THP as a tolerable, anthracycline-sparing neoadjuvant therapy option in patients with high-risk HER2+ eBC, Modi concluded.13
Broader Context
The expanding role of ADCs in the treatment of early-stage BC was the subject of several sessions at SABCS 2025. ADCs have demonstrated significant improvements in efficacy compared to single-agent chemotherapy and, as a class, are increasingly moving from metastatic BC (MBC) to earlier disease stages, with the aim of securing better long-term outcomes and even cures.14-16
ADCs are more effective than naked chemotherapy because they act as a payload reservoir, delivering sustained drug concentrations over time in the body and at the tumor site.15 Linker instability has proven to be essential for optimal ADC efficacy.15 In contrast to T-DM1, T-DXd employs a cleavable linker and also exerts a bystander effect that can overcome issues with HER2 heterogeneity.14,15 Collectively, these mechanistic differences may help to explain its outperformance of T-DM1 in DESTINY-Breast05, as well as the four-fold improvement in progression-free survival versus T-DM1 observed in patients with HER2+ MBC.14,17
However, despite advances in overall BC management with ADCs, there has been no improvement in rates of first disease recurrence in the central nervous system (CNS).14 In fact, prolonged survival from recent advances in MBC treatment has inadvertently provided more time for brain metastases to develop.18 Brain metastases can develop despite effective systemic disease control because the blood–brain barrier limits drug penetration, allowing intracranial tumor cells to proliferate unchecked.18 ADCs have variable mechanisms of release dependent on the linker, which impact their CNS delivery.18 Some encouraging early CNS signals of intracranial activity have been seen for ADCs with cleavable linkers such as sacituzumab govitecan and T-DXd.18 In the Phase IIIb/IV DESTINY-Breast12 study, the overall response rate in the CNS was approximately 70% in patients with measurable CNS disease treated with T-DXd, and most intracranial responses were ongoing at the time of data cutoff.18,19 Moving forward, patients with active BC brain metastases should be included in clinical trials of ADCs to further evaluate this emerging therapeutic potential.18
The pace of clinical development in HER2+ eBC is continuing to accelerate, and other notable presentations at SABCS 2025 included updates on the next-generation, site-specific, anti-HER2 ADC ARX788.20,21 In the multicenter, platform-randomized trial, I-SPY2.2, sequential ARX788 followed by standard anti-HER2 therapy demonstrated high efficacy, with a 63% complete response rate in the neoadjuvant BC setting, albeit with high ocular toxicity. The ISPY2.2 trial itself has demonstrated how response-adaptive neoadjuvant therapy can be tailored to each individual patient.20 In the area of risk stratification and real-time monitoring, results from a substudy of the PHERGain study showcased at SABCS showed a significant correlation between circulating tumor DNA clearance and pCR in HER2+ eBC undergoing neoadjuvant HER2-targeted therapy. Notably, detectable circulating tumor DNA at baseline was associated with inferior 3-year outcomes.1
Implementation Considerations
Appropriate and targeted patient selection is likely to prove key to maximizing the efficacy of ADCs in clinical practice, both in adjuvant and neoadjuvant eBC settings.14 Cross-comparison across clinical studies of ADCs in HER2+ BC suggests a correlation of pCR rate with hormone receptor status, tumor size, and treatment duration.14 Where T-DXd is concerned, the application of sensitive and objective HER2 assays in clinical trials could help to pinpoint the threshold expression level required for optimal T-DXd response.22 Multiplexed biomarker assays are also in development, which may potentially facilitate patient-centered use of ADCs in clinical management.23
As oncologists are well aware, T-DXd carries a rare but serious risk of ILD, necessitating frequent imaging and symptom evaluation.24 Current guidelines recommend permanent T-DXd discontinuation for Grade >2 ILD and temporary hold, with the option of rechallenge after resolution of imaging findings, for asymptomatic Grade 1 ILD.24 Results from a single-center, real-world study presented at SABCS 2025 showed that T-DXd rechallenge was associated with a long duration of clinical benefit and low rates of recurrent ILD, with no Grade 5 events.24 Also presented at SABCS was a meta-analysis looking at incidence and predictors of ILD associated with ADCs in BC, which found that payload was the strongest predictor of ILD risk and that ARX760 had the highest overall ILD risk among ADCs.25
With any proposed changes to the SOC based on emerging trial data, it is always important to consider the potential impact on real-world community oncology practice. Workflow and logistical issues must therefore be anticipated and addressed to streamline the incorporation of new treatment approaches into routine clinical care and maximize chances of success. Key factors to consider with T-DXd include patient monitoring and screening requirements (e.g., chest CT, etc.), pre-treatment medications, and appropriate implementation of AE management guidelines.
What Next?
As the landscape of eBC treatment continues to shift, clinical guidelines must evolve to align with new insights and understanding. Additional regulatory approvals will also be required to expand the licensed indications for current therapies into different disease settings, in particular, the movement of HER2-directed ADCs into the eBC space. During expert discussions at SABCS, Loibl suggested that future iterations of ESMO guidelines may evolve to differentiate between intermediate and high-risk patients with non-pCR HER2+ eBC who have residual disease. The former will likely continue to receive SOC T-DM1, while the latter may be treated with up to 14 cycles of T-DXd. RT and endocrine therapy can continue to be given, either concomitantly or sequentially to the anti-HER2 therapy.14
For the DESTINY-Breast05 study specifically, further analysis is needed to assess the impact of potential confounders such as race and comorbidities on ILD and RP risk. To maximize safety in clinical practice, it will also be essential to understand the influence of regional variability in RT use and different steroid management practices on the onset, duration, and outcomes from ILD/RP.9 Longer-term follow-up from both the DESTINY-Breast05 and 11 studies is important to continue to evaluate IDFS outcomes for T-DXd-treated patients and ultimately capture data on overall survival. Ongoing screening of patients over time will also shine a spotlight on the evolution of ILD risk with T-DXd over longer-term treatment.10
Conclusion
Results from the DESTINY-Breast05 and DESTINY-Breast11 studies were key highlights in HER2+ eBC at SABCS 2025, illustrating the potential of T-DXd to improve outcomes in curative-intent settings in eBC.
Findings from DESTINY-Breast05 could help to redefine T-DXd as a new post-neoadjuvant standard in HER2+ eBC, replacing T-DM1 in high-risk patients with residual invasive disease.10 Equally, results from DESTINY-Breast11 support T-DXd alone or followed by THP as an effective anthracycline-sparing strategy with manageable safety, signaling a potential shift toward ADC-based neoadjuvant regimens in high-risk HER2+ eBC.10,13
Moving forward, careful patient selection, toxicity management, and long-term data will be key to optimizing the therapeutic potential and positioning of ADCs in the management of HER2+ eBC across both the neoadjuvant and adjuvant settings.