Meeting Summary
Treatment for advanced non-small cell lung cancer (NSCLC) with activating mutations in HER2 relies on chemo-immunotherapy in the first-line setting, which fails to address the underlying human epidermal growth factor receptor 2 (HER2)-driven biology. This unmet need is fueling a shift toward HER2-targeted therapies for this patient population and for earlier lines of therapy. The European Society for Medical Oncology (ESMO) Congress 2025, held in Berlin, Germany, from October 17th–21st, included discussions of recent advances in the first-line treatment of HER2-mutant NSCLC. Among the highlights was the presentation of first-line data from the Beamion LUNG-1 Phase Ib expansion study evaluating zongertinib, an oral, HER2-selective tyrosine kinase inhibitor (TKI), in treatment-naive patients with advanced HER2-mutant NSCLC. The study demonstrated a confirmed objective response rate (ORR) of 77% by blinded independent central review (BICR), with a median time to response of 1.4 months. The study also demonstrated early signs of durability, with a 6-month duration of response (DoR) rate of 80% and a 6-month progression-free survival (PFS) rate of 79%. The safety profile of first-line zongertinib was manageable, with mostly low-grade treatment-related adverse events (TRAE) and a low incidence of interstitial lung disease (ILD)/pneumonitis (3%, both Grade 2). Additionally, ESMO 2025 featured data from SOHO-01, which evaluated another oral HER2 TKI, sevabertinib, in treatment-naive patients, further signaling a shift in the first-line treatment of HER2-mutant NSCLC from chemotherapy with or without immunotherapy to HER2-targeted therapies.
Introduction
Although relatively uncommon, NSCLC harboring mutations in HER2, also known as ERBB2, is a clinically distinct lung cancer subtype with unmet clinical needs.1 HER2 mutations occur in 2–4% of patients with NSCLC, and most activating HER2 mutations are found in the tyrosine kinase domain (TKD).2,3 HER2-mutant NSCLC is an aggressive lung cancer subtype with a poor prognosis and high rates of brain metastases, which are present in approximately 30% of patients at diagnosis and 47% during the disease course.2,4-6
In the USA, the current standard of care for treatment-naive patients with advanced HER2-mutant NSCLC is the same as that for those without activating HER2 mutations, i.e., platinum-based chemotherapy with or without immune checkpoint inhibitors.7,8 However, this approach does not address the underlying HER2-driven biology, and treatment outcomes are poor.9 In the Phase III KEYNOTE-189 trial, pembrolizumab plus chemotherapy in treatment-naive patients with metastatic NSCLC resulted in a 12-month overall survival rate of 69.2% and a median PFS of 8.8 months.9 However, KEYNOTE-189 trial participants were not selected for HER2 mutations, and the efficacy and safety of chemo-immunotherapy specifically in patients with HER2-mutant NSCLC have not been explored. Recent data presented at ESMO 2025 show progress in addressing this unmet need, with HER2-targeted TKIs being evaluated for first-line treatment of HER2-mutant NSCLC.6,10
In his presentation, Popat explained the rationale for testing zongertinib in the first-line setting.6 Zongertinib is an irreversible TKI that selectively inhibits HER2 while sparing wild-type epidermal growth factor receptor (EGFR).11 In previously treated patients with HER2-mutant NSCLC, zongertinib demonstrated a manageable safety profile and clinically meaningful efficacy, including activity in patients with brain metastases.12 Popat noted that zongertinib represents a targeted approach that could potentially improve outcomes compared to non- targeted chemo-immunotherapy regimens. The Beamion LUNG-1 study was designed to evaluate this hypothesis, with the first public disclosure of first-line data presented at the ESMO Congress 2025.6
First-Line Zongertinib for Advanced HER2 (ERBB2)-Mutant Non-small Cell Lung Cancer: Insights from BEAMION LUNG-1
Study Design and Patient Population
Beamion LUNG-1 is an ongoing Phase Ia/Ib dose expansion study evaluating zongertinib in patients with advanced solid tumors and HER2 alterations.13 Following Phase Ia dose escalation, where the maximum tolerated dose was not reached at 360 mg once daily, the selected dose for the Phase 1b expansion cohorts was 120 mg once daily after interim futility analysis.6,13 The ESMO 2025 presentation focused on Cohort 2, which included treatment-naive patients with HER2 TKD activating mutations.6
Key inclusion criteria were age ≥18 years, advanced or metastatic non-squamous NSCLC with HER2 TKD activating mutations, at least one measurable non-central nervous system lesion per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), and Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1.6 Patients with stable or asymptomatic brain metastases were eligible for inclusion.6
At the data cut-off of May 8th, 2025, 74 patients had received first-line zongertinib 120 mg once daily.6 The median age was 67 years (range: 35–88), gender distribution was balanced (50% female), and 45% of patients were non-Asian.6 Approximately one-third (35%) had a history of tobacco exposure, and 30% had baseline brain metastases.6 A majority of patients (66%) harbored the YVMA insertion (A775_G776insYVMA) mutation, and 32% had other HER2 TKD mutations.6
First-Line Efficacy Results Suggest Antitumor Activity
The primary endpoint of the study was objective response assessed by BICR using RECIST 1.1. The confirmed ORR was 77% (95% CI: 66–85%; p<0.0001 versus null hypothesis of ORR ≤40%), comprising 8% complete responses and 69% partial responses.6 Waterfall plot analysis revealed that tumor shrinkage occurred across all patients, irrespective of HER2 mutation subtype (YVMA versus other HER2 TKD activating mutation; Figure 1).6
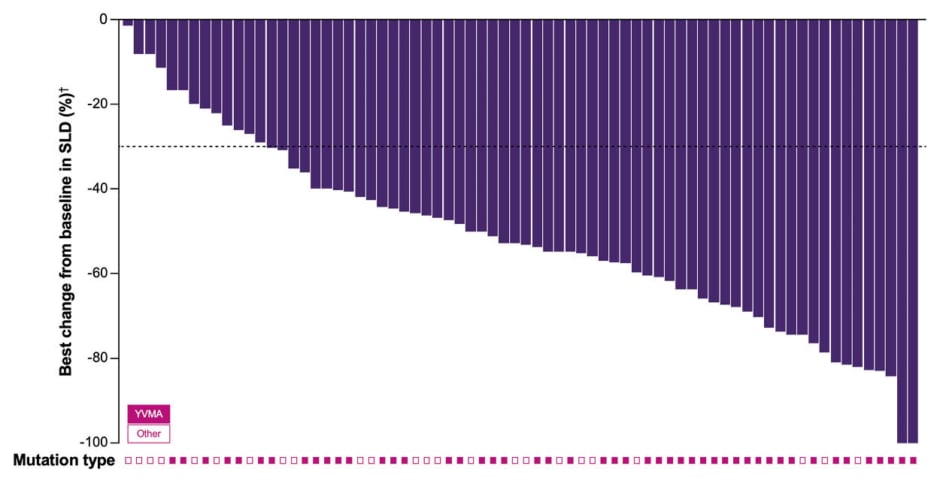
Figure 1: Best percentage change from baseline in target lesion diameter in treatment-naive patients with advanced non-small cell lung cancer harboring HER2 TKD activating mutations who received zongertinib 120 mg once daily (N=74).*,6
*Two patients were not evaluable for response (images were not available).
?Due to the central review process, lesion measurements are available for investigator assessment.
Each bar represents an individual patient. The confirmed ORR rate by BICR (RECIST v1.1) was 77% (95% CI: 66–85%), with 8% complete responses and 69% partial responses.
BICR: blinded independent central review; SLD: sum of the longest diameters of lesions; ORR: objective response rate; RECIST v1.1: Response Evaluation Criteria in Solid Tumors version 1.1; TKD: tyrosine kinase domain.
The disease control rate, a key secondary endpoint assessed by BICR, was 96% (95% CI: 89–99%), with 19% of patients achieving stable disease.6 One patient (1%) experienced progressive disease as best response, which was due to non-target lesion progression.6 Popat noted that responses to zongertinib were rapid, with most observed at the first assessment (Figure 2). The median time to objective response was 1.4 months (range: 1.1–6.9).6
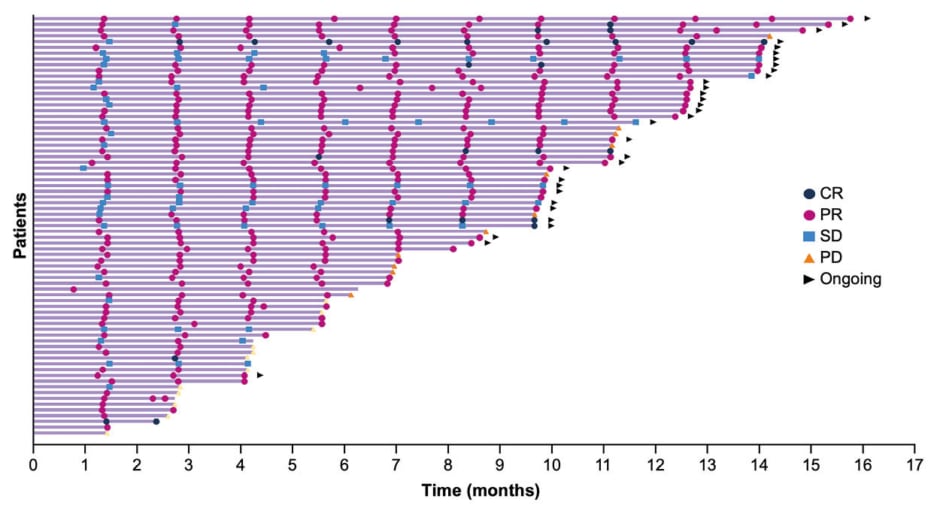
Figure 2: Durability of response in treatment-naive patients with advanced non-small cell lung cancer harboring HER2 TKD activating mutations who received zongertinib 120 mg once daily (N=74).*,6
*Two patients were not evaluable for response (images were not available).
Each bar represents an individual patient. With a median follow-up of 9.7 months (95% CI: 7.1–9.9), the median time to objective response was 1.4 months (range: 1.1–6.9).
CR: complete response; PD: progressive disease; PR: partial response; SD: stable disease; TKD: tyrosine kinase domain.
First-Line Efficacy Results Suggest Durable Responses
Secondary endpoints included DoR and PFS, both of which were assessed by BICR.6 At data cut-off, 47% of responding patients remained on treatment, indicating sustained benefit from zongertinib. The 6-month DoR rate was 80% (95% CI: 65–89%), and the 6-month PFS rate was 79% (95% CI: 68–87%).6 Median DoR and median PFS were not yet reached at the time of analysis.6
Case Studies
During his talk, Popat presented two cases that illustrate the rapid and durable antitumor activity of zongertinib in treatment-naive patients with HER2-mutant NSCLC, including those with central nervous system involvement.6 The first involved a 69-year-old female former smoker with metastases in the bones and lungs who had the YVMA mutation. The patient showed a durable partial response from the first post-treatment CT scan, with continued tumor shrinkage beyond 20 weeks.6
The second case was a Stage IV lung adenocarcinoma in an 85-year-old female who had never smoked.6 The patient had the uncommon V777L mutation and brain metastases at baseline. She experienced rapid systemic response across nearly all metastatic sites, including intracranial response demonstrated on brain CT scan at 6 weeks.6
Manageable Safety Profile with Low-Grade Toxicity
The safety profile of first-line zongertinib was generally manageable and consistent with previously reported data from pretreated patients.6,12 TRAEs were reported in 67 patients (91%), but most were low-grade (Figure 3). Grade 3 TRAEs occurred in 13 patients (18%), and, as of the data cut-off, no Grade 4 or 5 TRAEs were reported.6
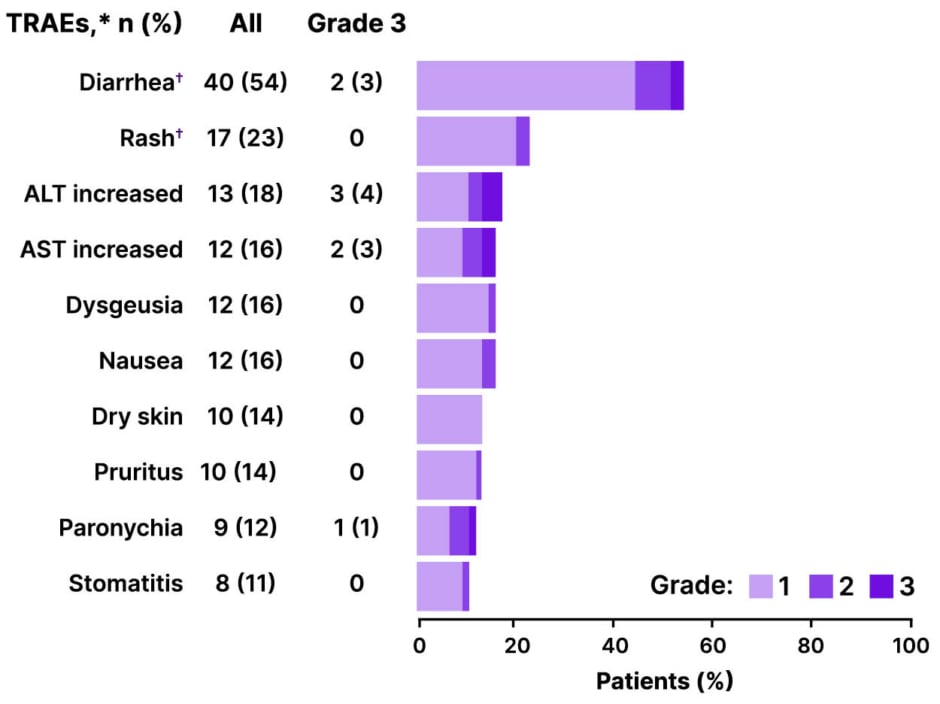
Figure 3: Safety profile of zongertinib in treatment-naive patients with advanced non-small cell lung cancer harboring HER2 TKD activating mutations.6
*TRAEs as assessed by the investigator that occurred in ≥10% of patients are shown.
?Grouped terms.
ALT: alanine aminotransferase; AST: aspartate aminotransferase; TRAE: treatment-related adverse events.
The most common TRAEs were diarrhea (54% any grade; 3% Grade 3), rash (23% any grade; 0% Grade 3), increased alanine aminotransferase (ALT; 18% any grade; 4% Grade 3), increased aspartate aminotransferase (AST; 16% any grade; 3% Grade 3), dysgeusia (16% any grade; 0% Grade 3), and nausea (16% any grade; 0% Grade 3).6
Popat noted that the low rates of severe diarrhea, rash, paronychia, and stomatitis likely reflect the fact that zongertinib spares wild-type EGFR, distinguishing it from pan-HER inhibitors or EGFR TKIs that can cause high-grade gastrointestinal and dermatological toxicities.6,14
ILD or pneumonitis, a toxicity of concern for patients with lung cancer,15 was reported in two patients (3%). Both cases were Grade 2 and fully resolved.6 Despite the low incidence of ILD or pneumonitis, vigilance for pulmonary symptoms is essential, particularly for high-risk patients.15
Adverse events leading to dose reduction occurred in 11 patients (15%).6 The most common reasons were ALT or AST elevation (both; n=3), diarrhea, and decreased ejection fraction (both; n=2).6 Adverse events leading to treatment discontinuation occurred in seven patients (9%), including ejection fraction decrease (n=2), ALT increase, anemia, AST increase, diarrhea, pericardial effusion, and pneumonitis (all; n=1).6 The median duration of treatment was 10.3 months (range: 0–16.0), indicating that most patients were able to remain on therapy for extended periods.6
SOHO-01: Sevabertinib in the First-Line Setting for HER2-Mutant NSCLC
ESMO 2025 also featured updated results from the SOHO-01 study, a Phase I/II trial evaluating sevabertinib, an oral, reversible TKI that binds to both HER2 and EGFR, in patients with HER2-mutant NSCLC.10 Cohort F of SOHO-01 included 73 treatment-naive patients who received sevabertinib 20 mg twice daily.10,16 The baseline characteristics of SOHO-01 Cohort F differed somewhat from those of Beamion LUNG-1 Cohort 2.6,10 The median age was 65 years, 63% were female, and 70% were Asian.10 The rate of brain metastases at baseline was considerably lower in Cohort F of SOHO-01 (12%) than in Beamion LUNG-1 Cohort 2 (30%), and prior radiotherapy was required for all patients with baseline brain metastases in the SOHO-01 study.6,10
At the data cut-off of June 27th, 2025, the confirmed ORR by BICR was 71% (95% CI: 59–81%), and the disease control rate was 89% (95% CI: 80–95%).10 At a median follow-up of 9.9 months, median DoR was 11.0 months (95% CI: 8.1–not estimable), and median PFS was not estimable (95% CI: 9.6–not estimable). The 12-month PFS rate was 55%.10
Systemic response rates were generally similar in patients with and without baseline brain metastases. Among the nine patients with baseline brain metastases in Cohort F, the ORR was 78%, which is comparable to the ORR in the overall cohort (71%) and in patients without brain metastases (70%).10 Among patients without brain metastases at baseline, 3% developed new brain lesions as the site of first progression.10
Drug-related adverse events with first-line sevabertinib were reported in 97% of patients in Cohort F, including 21% with Grade ≥3 events.10 Diarrhea was the most common TRAE in all cohorts.10,16 In Cohort F, any grade diarrhea was reported in 84% of patients, and Grade 3 diarrhea occurred in 5% of patients.16 No cases of ILD or pneumonitis were reported.10
Broader Treatment Landscape for HER2 (ERBB2)-Mutant Non-small Cell Lung Cancer
Recent regulatory approvals underscore a broader shift in the treatment landscape for HER2-mutant NSCLC, moving from chemo-immunotherapy approaches toward precision medicine strategies that target oncogenic HER2 mutations. In 2022, the HER2-directed antibody–drug conjugate trastuzumab deruxtecan (T-DXd) was granted accelerated approval by the FDA for the treatment of patients with NSCLC bearing oncogenic HER2 mutations who had disease progression following systemic platinum-based therapy.17 On August 8th, 2025, zongertinib became the first orally administered HER2-selective TKI that received accelerated approval by the FDA for the treatment of patients with NSCLC harboring HER2 TKD activating mutations who have received prior systemic therapy; this decision was based on Beamion LUNG-1 Cohort 1 data.12,18,19 On November 19th, 2025, sevabertinib was also granted accelerated approval by the FDA for the treatment of locally advanced or metastatic, non-squamous NSCLC in patients with HER2 TKD activating mutations who have received prior systemic therapy.20
Despite these advances in the second-line treatment of HER2-mutant NSCLC, no targeted therapy has been approved for first-line treatment of NSCLC with HER2 activating mutations.7 The current standard of care for treatment-naive patients with HER2-mutant advanced NSCLC in the USA remains the same as that for patients without HER2 mutations, which is platinum-based chemotherapy with or without immunotherapy.7,8 Based on the first-line data from the Beamion LUNG-1 trial, the FDA has granted zongertinib Breakthrough Therapy Designation for the first-line treatment of adult patients with unresectable or metastatic NSCLC whose tumors have HER2 TKD activating mutations.21 In addition, the FDA has awarded a Commissioner’s National Priority Voucher (CNPV) for zongertinib, which is designed to expedite the regulatory review process while maintaining rigorous safety and efficacy standards.22 The ongoing Phase III Beamion LUNG-2 study aims to compare the efficacy and safety of first-line zongertinib versus standard of care (platinum-based chemotherapy with immunotherapy) in patients with unresectable, locally advanced, or metastatic NSCLC with HER2 TKD mutations.23 Similarly, T-DXd and sevabertinib are also being compared with the standard of care in the Phase III Destiny Lung-04 and SOHO-02 trials as first-line therapy for locally advanced or metastatic NSCLC with HER2 activating mutations.24,25 The Destiny Lung-04 trial is evaluating T-DXd in patients with NSCLC harboring HER2 exon 19 or 20 mutations, and the SOHO-02 trial is evaluating sevabertinib in patients with activating mutations in the HER2 tyrosine kinase domain.24,25
The shift from chemo-immunotherapy approaches to HER2-targeted TKIs for advanced NSCLC makes timely testing for HER2 mutations essential. Biomarker testing is recommended for all patients with newly diagnosed advanced non-squamous NSCLC to identify HER2 mutations and other actionable drivers.26
Conclusion
The Beamion LUNG-1 first-line data presented at ESMO Congress 2025 represent a step forward in addressing the unmet need for targeted therapy in treatment-naive patients with HER2-mutant NSCLC. Zongertinib demonstrated high response rates; rapid onset of benefit, encouraging early durability; and a manageable safety profile in treatment-naive patients.6 The first-line sevabertinib data from SOHO-01 presented at the Congress provide further support for the potential of HER2-targeted TKIs in the first-line setting.10