Interview Summary
Up to three-quarters of people with asthma have symptoms typically described as ‘mild’. However, such patients may not have their symptoms fully controlled and may experience dangerous exacerbations. Although the latest Global Initiative for Asthma (GINA) report recommends that all patients with asthma take low-dose inhaled corticosteroids (ICS) whenever a short-acting β2-agonist (SABA) is administered (either as a combination therapy or in separate inhalers), many are still prescribed SABA therapy alone, which is not recommended by GINA. While inhaled SABAs are highly effective for the quick relief of asthma symptoms, patients whose asthma is treated with SABA alone (compared to those taking an ICS, either as daily maintenance or as-needed in combination with SABA) are at an increased risk of asthma-related death and need for urgent asthma-related healthcare, even if they have good symptom control. Here, three experts in the field discuss evidence-based approaches to help patients living with mild asthma, along with the design of and results from the Phase IIIb BATURA trial. This trial investigated the efficacy and safety of as-needed use of an albuterol-budesonide (ALB-BUD) pressurized metered dose inhaler (pMDI) compared with albuterol sulfate (ALB) alone in adolescents ≥12 years and adults with mild asthma. The trial was fully decentralized and virtual, meaning all study procedures were done from home and patients did not have to give up time and money to travel to a study site to participate. The decentralized design also facilitated the inclusion of an ethnic and racial makeup of patients more representative of the US, which, the experts discussed, may not always be the case in clinical trials. However, the virtual design also meant that patients were not phenotyped, and that outcomes were reported remotely. Results included a significant 47% reduction in the annual risk of a severe exacerbation with ALB-BUD compared with ALB alone (p<0.001). The results also showed a reduction in the use of systemic glucocorticoids, use of which leads to adverse effects. As patients with mild asthma are typically seen by primary care practitioners (PCP), the experts agreed that the BATURA trial results potentially provide a simple way to help patients control symptoms and mitigate future adverse outcomes in the primary care setting using an as-needed ICS-SABA combination.INTRODUCTION
An estimated 50−75% of people with asthma have symptoms classified as ‘mild’, which has a worldwide prevalence of around 3.3%.1 The latest GINA strategy document summarizes current evidence to provide expert guidance on how to manage asthma. According to this report, the term ‘mild asthma’ may be used to denote a patient with mild or infrequent symptoms who is also assumed to be at low risk for exacerbations. However, the report questions the use of this term in clinical practice as “patients with infrequent symptoms can still have severe or fatal exacerbations.”2 To discuss issues around the manifestation and as-needed treatment of mild asthma with an ICS-SABA combination, AMJ sat down with three experts in the field: Njira Lugogo from the University of Michigan, Ann Arbor, USA; Monica Kraft from the Icahn School of Medicine at Mount Sinai, Mount Sinai Health System, New York City, New York, USA; and Reynold Panettieri from Rutgers Institute for Translational Medicine and Science, Rutgers, State University of New Jersey, New Brunswick, USA. The latter two were co-authors in the BATURA trial, a Phase IIIb, randomized, double-blind, parallel-group, virtual exacerbation study that investigated the efficacy and safety of the as-needed use of a single albuterol-budesonide pMDI in people aged ≥12 with mild asthma, compared with ALB alone.3
UNDERSTANDING MILD ASTHMA
According to Panettieri, typically in patients with mild asthma, “we don’t see the dramatic decrease in quality of life that we see with moderate–severe asthma, and they tend not to have the same number and magnitude of exacerbations.”4 This may mean, though Lugogo discussed, that for such patients, “there’s an assumption that a lack of symptoms means you don’t have anything going on, but,” she suggested, “we know from other chronic diseases that a lack of symptoms doesn’t mean there’s no disease pathology.”
Indeed, in an analysis including 4.5 million US patients, of the 86% classified as having mild–moderate asthma, 30% had uncontrolled disease.5 It is also of note that up to 40% of exacerbations requiring emergency care may be in patients classified as having mild asthma, with an estimated frequency of severe exacerbations between 0.12–0.77 episodes per patient per year.1 “What misleads the community,” said Kraft, “is that, [as] in between exacerbations patients are potentially relatively asymptomatic, these folks aren’t necessarily coming to the attention of healthcare providers and, if they are, it may only be at the time of exacerbations.” These findings indicate, said Lugogo, that “we need to focus on how to mitigate risk, identify people at high risk, and treat them appropriately.” Kraft agreed, adding that “we want to be more like the cardiologists, treating blood pressure to prevent cardiac events.”
One way Kraft discussed she’s doing this is by “trying to move asthma up on PCPs’ radar, because I think that sometimes it gets forgotten.” She explained how, by helping PCPs appreciate the potential severity of even mild asthma, “they can be proactive and make a huge difference in their patients’ lives.” Furthermore, Kraft is currently recruiting patients with mild asthma for a study that includes training PCPs on how to best identify such patients through spirometry and biomarkers. “By participating in something like this,” she said, “I’m hoping it will really get [PCPs] thinking about how asthma is not always mild … and maybe the fact that these patients are [also] getting one or two courses of oral corticosteroids (OCS) a year is not acceptable.”
SABA-ONLY USE IN PATIENTS WITH MILD ASTHMA
The latest GINA report recommends against SABA-only treatment (SABA use without using ICS).2 However, the US analysis discussed above, including over 4.5 million patients with mild–moderate asthma, found that 20% were on SABA-only therapy.5 In the BATURA trial, around three-quarters of the patients with mild asthma entering the study were on SABA-only treatment, despite 8% of this cohort having had ≥1 severe exacerbation in the previous year.3
SABA-only rescue therapy, explained Panettieri, “treats only one aspect of the disease, symptom-driven bronchoconstriction (chest tightness, coughing, wheeze), but it doesn’t really treat the underlying inflammatory component.”2,6 Lugogo echoed this, discussing how “one thing we haven’t done well is really emphasize the pathophysiology of the disease and then tie the treatment to this pathophysiology. It’s never made much sense to treat bronchoconstriction only. Often the inflammatory pathways are driving the bronchoconstriction, so they are interrelated.2,7 When you explain that to patients, they really do get that this is a disease that has two processes, and we have to target them concomitantly.” Panettieri discussed though that, historically in the US (before the approval of albuterol/budesonide), there was “no opportunity to include an ICS-SABA together, as it’s been excluded by the FDA.” This could be problematic,” he continued, “if a patient had a Type 2 inflammatory trigger that couldn’t be controlled by maintenance therapy.”2
With this in mind, Lugogo suggested that “we really need SABA stewardship and a paradigm shift.” For example, she discussed how prescribing unlimited SABA refills may be common practice even though, according to the GINA report, exacerbation risk can be increased by SABA overuse (three or more 200-dose canisters/year) with increased mortality at one or more canisters/month.2 Identifying patients may be aided by electronic medical records and examining refill rates, said Kraft, adding that “there’s a decision support approach that is triggered when a patient comes in and they’re on a SABA-only regimen, there’s a little flag that comes up and tells the provider, ‘hey, this person’s only on albuterol, think about this’.”
As such, there’s a need, said Lugogo, “to think about anti-inflammatory fast-acting relievers as the preferred treatment algorithm for all patients with asthma, and particularly the mild patients, because they are at quite a significant risk.” She discussed how, in her practice, “if a patient is newly diagnosed with asthma, I never introduce the idea of SABA monotherapy, I just say, ‘you’re going to be on an anti-inflammatory fast-acting reliever strategy, and these are your options’.”
THE BATURA TRIAL: DESIGN AND INNOVATION
The GINA report states that asthma management should include controlling both symptoms and future risk of adverse outcomes. They recommend that patients with ≥1 exacerbation risk factor or ≥1 severe exacerbation in the previous year use as-needed or daily maintenance ICS/formoterol or, as an alternative, an ICS- SABA combination, depending on symptom frequency and lung function.2
The goals of the BATURA trial, explained Kraft, “were to identify a group with mild asthma who were not on an anti-inflammatory therapy or were on low dose ICS therapy, treat them with an as-needed ICS-SABA, and look at [the] change in exacerbation rate.” The trial included 1,209 in the ALB-BUD group compared with 1,212 in the ALB-only group. While adolescents ≥12 years were eligible, 97.2% of participants were ≥18 years. Patients were stratified by baseline SABA-only (74.4%) or ICS-SABA (25.6%) use and exacerbations in the previous year (0, 1, 2, or >2; Figure 1).3
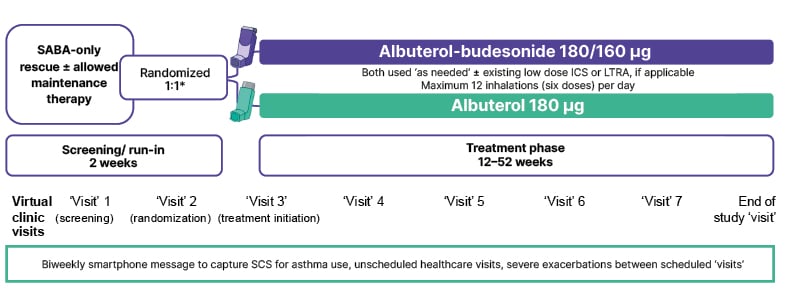
Figure 1: BATURA trial design.3
*Participants stratified by pre-study asthma medication and number of prior severe exacerbations (0, ≥1) in the 12 months prior to the screening visit.
Adapted from LaForce et al.3
ICS: inhaled corticosteroid; LTRA: leukotriene receptor antagonist; SABA: short-acting β2-agonist; SCS: systemic corticosteroid.
“The fact that we reached down into the intermittent mild asthmatic [patient] to address the benefit of an ICS-SABA was unique,” said Panettieri. “These patients are not typically enrolled in studies where specialists run clinical trials in moderate-to-severe asthma.” He also highlighted how “what we’ve done is embrace a group of individuals, cared for predominantly by PCPs, who had an unmet need because the disease was not controlled.”
Lugogo underscored how the design of the BATURA trial, in which therapy was only used as-needed, was “a very patient-centric treatment approach. It gives them a lot more control. For the vast majority of patients, it follows the disease trajectory, which is when you have an exposure, your inflammation increases, you increase anti-inflammatory therapy on demand, and you get through it without going on to develop a severe exacerbation,” she added. This means, she continued, that patients “don’t have to commit to a medication all the time, they can take it when they need it, and if they do, it works.”
Of note, the BATURA trial was entirely virtual, decentralized, and event driven.3 Kraft discussed how “this virtual approach [recruited] participants who may not normally be very engaged in [clinical trials].” Panettieri highlighted how “doing studies in homes [and] reaching out to patients where they live dramatically improved the ethnic and racial makeup of the study to more closely mirror the US population.” This study design was also important, he explained, as it meant “you’re not relying on a patient to go to a study site or expend money and time. All those things become obstacles … especially for low-income families or others who can’t take time off from work. The only way you can mitigate that challenge is to do the study in their house, at a time when they can do it. You’re not compelling someone to be at a study visit at a particular time that’s convenient for the staff and not for the patient.”
However, “the challenge,” said Kraft, “is that we don’t have as much information on these patients as we’d love to have. Typically, in asthma clinical trials, we do a lot of phenotyping that has to be done in a study visit in person, so we lose a little bit of that.” Panettieri agreed, discussing that, while BATURA did include valuable patient reported outcomes and recorded exacerbations, “it would have been a logistical challenge for us to do high quality spirometry in patient’s homes.” He also noted how “to do a virtual study requires an enormous number of logistical challenges and bottlenecks that [have] to be overcome.”
INSIGHTS ON BATURA TRIAL RESULTS
Previously, the MANDALA trial showed that as-needed use of the same ALB-BUD therapy evaluated in BATURA could improve outcomes above the standard of care in patients with moderate-to-severe asthma.8 For the patients in the BATURA trial with mild asthma, as-needed use of ALB-BUD led to a significant (p<0.001) 47% reduction in the percentages experiencing a severe exacerbation (5.1%) compared with ALB use alone (9.1%), with a hazard ratio of 0.53 [95% CI: 0.39–0.73; p<0.001]. ALB-BUD use reduced the annualized exacerbation rate compared to ALB by 53% (0.15 versus 0.32; rate ratio: 0.47; 95% CI: 0.34–0.64). Both treatments were generally well-tolerated, with similar between-group adverse event incidence and type. Notably, due to the significant difference in efficacy between the groups at a prespecified interim analysis, the trial was terminated early.3
The significant decrease in the number of exacerbations in patients with mild asthma shown here was interesting, said Panettiere, as it was “in people who we didn’t think had a lot of exacerbations.” This means that “what we were able to do is extend [ICS-SABA therapy] into a group of individuals who may not be so affected by the disease and have a profound effect on outcomes.” The BATURA trial results also add to the evidence that patients with mild asthma can benefit from an ICS-SABA combination when taken as needed, as opposed to on a daily regimen. “That works,” said Kraft, “because [patients] don’t always want to be on a schedule of medications. That’s been a real barrier to effectively treating [them because], when they feel better, patients often think, ‘maybe I don’t need these medications, maybe I’ll just stop them, I don’t need this for my whole life’.”
In a Europe-based study including the comparison of patients with mild asthma and those who had never experienced asthma, health-related quality-of-life indices were lower in the former, and there was a significant increase in work absenteeism and activity impairment.4 With regard to the BATURA trial results, Kraft said that “if we think about … quality of life, reducing future risk of exacerbations, ability to do activities of daily living and to live a normal life, adding this regimen is really moving patients in that direction.” She additionally highlighted reductions in the 25% of patients already taking low-dose ICS.3 “I was impressed that [this] group still saw benefit, because one might think, ‘they’re already getting anti-inflammatory therapy, so why would adding a bit more matter?’ Well,” she suggested, “patients don’t always take their ICS as they should on a standard regimen every day; they might miss doses sometimes. Adding this additional anti-inflammatory approach allowed them to have even greater benefit.”
Another finding was that mean annualized total exposure to systemic glucocorticoids was 62.5% lower with ALB-BUD compared to ALB (23.2 versus 61.9 mg/year; p<0.001).3 This is of note, as the US study including 4.5 million people with asthma found that 15% had ≥2 systemic corticosteroid prescriptions annually.5 Lugogo discussed how, historically, “we’ve not done tremendously well talking about the harms associated with OCS.” This is despite the findings of, for example, a 2018 observational study of adult patients with active asthma that found a significantly increased risk of myriad adverse events in patients prescribed systemic corticosteroids compared with those not receiving such medications. These included osteoporosis/osteoporotic fracture, pneumonia, cardio/cerebrovascular diseases, cataracts, sleep apnea, renal impairment, depression/anxiety, Type 2 diabetes, and weight gain. Adverse outcomes occurred at cumulative exposures of only 500–1,000 mg, with a dose-response relationship for cumulative exposure and adverse outcomes.9 As such, Lugogo described how “protecting patients from OCS toxicity and embracing OCS stewardship is really important.”
IMPLEMENTING FINDINGS FROM THE BATURA TRIAL
In an article where the BATURA trial results were published, it was noted that “we should be working toward a world in which all patients with asthma are easily able to leave home with a combination inhaler in their pocket’ and said of the BATURA trail results that they ‘now provide the strong evidence needed for physicians to prescribe this [ICS-SABA] regimen for patients with mild asthma.”10 The simplistic design of the BATURA trial was notable,3 said Lugogo, because typically in clinical research trials “we need … to have very high standards about who gets in, but often times, implementation of those findings is quite onerous in practice. For example, if you’re going to try to implement a process for seeing patients, measuring lung function, doing all the measures of inflammatory phenotypes, [thinking] about comorbidities, optimizing therapies, and deciding if they need a biologic, and starting the biologic, it’s quite complicated. There is a mental hurdle sometimes in embracing a treatment paradigm that seems very complicated, figuring out all these factors, then deciding who’s at risk, instituting a standard, twice daily therapy, following people very closely, figuring out what to adjust, it feels daunting.”
However, continued Lugogo, the BATURA trial design and outcomes mean that, if any patient with mild asthma is on SABA-only therapy, their rescue therapy can be simply switched to an ICS-SABA pMDI. “This kind of real-world design is much more generalizable to our clinical practice,” she explained, “so you can have confidence that you should be seeing similar, if not better, outcomes based on the study results and design.”
The design, Lugogo continued, is also applicable to primary care. “We’re not asking people to do a very detailed and complicated workup of these patients, we’re just saying, change this one treatment [and] we can make a tremendous difference, and we don’t have to know every GINA step and all the guidelines.” She also discussed how “there wasn’t intensive follow-up and reminders and measuring and things that make it seem like the treatment effect may be coming from other things that come along with clinical trial interventions.”
DIVERSITY, GENERALIZABILITY, AND EQUITY IN ASTHMA CARE
Talking about barriers to ICS-SABA therapy, Panettieri discussed how “the hurdles and chasms that have to be crossed [in the US] are substantial. The first, second, and third most important is access to the drug, not only having it in the pharmacy, but also paying for it. The downside in the US is that there [are] copayments, and substantial preauthorization is necessary. These are fundamental obstacles.”
Kraft agreed, adding that “I live in New York City and one of our hospitals is juxtaposed between some of the wealthiest ZIP codes in the country and some of the least wealthy. We serve an extremely diverse population and access to medications is really important. One issue with the ICS-SABA was that coupon cards to reduce the copay are not available to patients who have Medicaid. That is changing in that there’s much more coverage now for all patients, but it’s taken a little while to get us there.”
Another issue, said Lugogo while discussing her clinical practice, is that “in Michigan, because most people’s insurance will cover an ICS and the albuterol, I had been [prescribing] two separate inhalers, which is less ideal [and] requires me to tell patients ‘you have to take these at the same time, that is an interim step you can take while we wait for formularies to catch up’.”
TAKE HOME MESSAGES
Wrapping up, Panettiere discussed how “in the US, for the first time in over 50 years, we have a novel rescue therapy that can help patients in a real manner, not only feel better and relieve their symptoms, but also improve the outcomes of exacerbations [that] could render some individuals irreversibly obstructed and have a long- term consequence on their health.”
Kraft highlighted how the BATURA trial results help focus on how important it is, in line with the GINA report,2 not to follow a SABA-alone approach for patients with mild asthma. These results, she said, help PCPs “think about their milder patients and what they can do for them. That is something very tangible and will make a huge difference.”
“We also need to start thinking about clinical trial innovation [and] our treatment paradigms,” concluded Lugogo. “I feel like the innovation that people embraced during COVID has slowly whittled away. BATURA was a very well executed study run fully remotely, but there are almost none [with a similar design] going on right now. We’ve gone back to being fully on site, gathering every little piece of information we can possibly get our hands on and controlling everything. We’ve proved [the virtual design of the BATURA trial] works, so we could have more confidence and do more decentralized trials.”







