Meeting Summary
At the American Thoracic Society (ATS) 2025 International Conference in San Francisco, California, USA, speakers presented data on nerandomilast (BI 1015550), an investigational oral preferential phosphodiesterase 4B (PDE4B) inhibitor, in patients with idiopathic pulmonary fibrosis (IPF) and progressive pulmonary fibrosis (PPF).
Phase III results from the FIBRONEER™-ILD (n=1,176) and FIBRONEER™-IPF (n=1,177) trials were featured across multiple sessions. Both studies met their primary endpoint, with nerandomilast (9 mg and 18 mg twice daily) significantly reducing the rate of forced vital capacity (FVC) decline over 52 weeks, irrespective of background antifibrotic use. Nerandomilast demonstrated a favourable safety profile, with a numerical reduction in the risk of the key secondary endpoint (KSE): time to first acute exacerbation, hospitalisation for respiratory cause, or death, over the duration of the trial. A nominally significant reduction in the risk of death was observed in patients receiving nerandomilast compared to placebo.
In additional analyses, nerandomilast delayed the initiation of supplemental O2 in patients with IPF, though further research is required to evaluate long-term benefits. Additional studies indicated that nerandomilast can be used in patients with mild or moderate renal and hepatic impairment without the possible need for dose adjustment, even though nerandomilast was found to be metabolised through the CYP3A4 pathway. Mechanistic studies indicated that PDE4B inhibition may contribute to the modulation of pro-inflammatory and fibrotic pathways, although further investigation is needed to confirm these mechanisms in clinical settings.
Together, these findings contribute to the evolving evidence base on nerandomilast and its potential role in the management of interstitial lung diseases (ILD).
Interstitial Lung Diseases
The most common chronic progressive fibrosing ILD is IPF.1,2 PPF is another form of progressive fibrotic lung disease that occurs in ILDs other than IPF.3 Both IPF and PPF are characterised by progressive fibrosis, declining lung function, dyspnoea, and high mortality rates.4,5
The treatment landscape of ILD has evolved since the introduction of therapies targeting the growth and migration of scar-forming fibroblasts.6 Currently, nintedanib and pirfenidone are approved for the treatment of IPF, while nintedanib alone is approved for PPF and progressive fibrosing-ILD.7,8 Both of these drugs help slow the decline in lung function.7,8 However, the disease continues to progress, with an average life expectancy of 3–5 years after initial diagnosis,9,10 and these therapies can cause adverse effects11,12 that lead to treatment discontinuation.12
There is an unmet need for more effective treatments for both IPF and PPF. Over the past decade, several Phase II and III trials in ILD have failed, underscoring the challenges of drug development.13 Novel differentiated therapies should address the complexity of IPF pathobiology by targeting multiple cell types and pathways.6
Targeted Therapy for Interstitial Lung Diseases and the Role of Nerandomilast
This article summarises findings presented at ATS 2025 on nerandomilast (BI 1015550), an orally administered preferential inhibitor of PDE4B that exhibits antifibrotic and immunomodulatory properties.14,15
FIBRONEER-IPF and FIBRONEER-ILD
Trial Design
FIBRONEER-ILD and FIBRONEER-IPF shared the same overall design, with treatment continuing until the end of the trial.16,17 The primary endpoint was absolute change from baseline in FVC (mL) at Week 52. The KSE was time to first acute exacerbation, hospitalisation for respiratory cause, or death, over the duration of the trial.16 Acute exacerbations and hospitalisations for respiratory cause were investigator-reported and not adjudicated.16,17
The trials had two database locks: the first (DBL1) occurred after the last patient completed the Week 52 visit, and the second (DBL2) took place once all patients completed their end-of-treatment visit. In FIBRONEER-ILD, Maher reported a mean treatment exposure of 14.4±5.3 months at DBL1.16 In FIBRONEER-IPF, Richeldi highlighted DBL2 as the most complete dataset, capturing a greater number of events due to longer observation. Mean treatment exposure was 13.3±4.4 months at DBL1 and 14.8±5.1 months at DBL2.17
Inclusion Criteria
FIBRONEER-ILD included patients ≥18 years old with a diagnosis of ILD other than IPF, and who were required to have a minimum level of lung involvement, with >10% fibrotic involvement on high-resolution CT ≤12 months prior to screening and ≥1 pre-defined criterion for progression within the previous 24 months.16 In FIBRONEER-IPF, eligible patients were ≥40 years old with a confirmed diagnosis of IPF based on updated 2022 international guidelines5 and a usual interstitial pneumonia (UIP) or probable UIP pattern based on high-resolution CT.17 Both trials required a FVC ≥45% predicted, and a mean diffusing capacity of the lung for carbon monoxide (DLCO), corrected for haemoglobin ≥25% predicted.16,17
Recognising that both trials included patients with advanced ILD, background standard-of-care treatment was allowed to continue. Patients were either required to be on stable therapy for ≥12 weeks prior to screening or off antifibrotic therapy for ≥8 weeks prior.16,17 In FIBRONEER-ILD, only nintedanib background therapy was included;2,3,14,16,17 and in FIBRONEER-IPF, background therapy included nintedanib or pirfenidone.
Baseline Characteristics
FIBRONEER-ILD enrolled 1,176 patients with a fibrosing ILD other than IPF.16 The most common diagnoses were autoimmune disease-related ILD (27.6%, including rheumatoid arthritis- and systemic sclerosis-associated ILD and mixed connective tissue disease), hypersensitivity pneumonitis (19.8%), unclassifiable idiopathic interstitial pneumonia (19.6%), idiopathic nonspecific interstitial pneumonia (19.4%), and other progressive fibrosing ILDs (13.5%) such as sarcoidosis. Mean baseline FVC was 70.1±15.8% predicted, DLCO was 49.3±16.9% predicted, and 71.4% of patients had a UIP or UIP-like fibrotic pattern.16 In FIBRONEER-ILD, 43.5% of patients were taking background nintedanib at baseline.16 Maher reported that the baseline demographics were broadly similar to FIBRONEER-IPF, though the population was slightly younger (mean age of 66.4±10.0 years) and had a more evenly distributed sex (55.6% male), and the mean time since diagnosis was approximately 4.2 years.
FIBRONEER-IPF enrolled 1,177 patients (mean age of 70.2±7.7 years; 83% male) with a confirmed IPF diagnosis. Mean baseline FVC was 78.2±17.3% predicted, and DLCO was 50.9±16.3% predicted.17 Richeldi described these characteristics as typical of the IPF population, with a mean time since diagnosis of around 3.5 years.17 Most patients (77.7%) were taking background antifibrotic therapy (45.4% nintedanib and 32.3% pirfenidone).17 Richeldi noted that patients taking background antifibrotic therapy had a longer disease duration and more severe disease compared with those not on background therapy. For example, Wijsenbeek presented data for FIBRONEER-IPF, where time since diagnosis was 3.7 years for those on background therapy versus 2.8 years in those without, and baseline FVC was lower (77.1 versus 82.2% predicted, respectively). Wijsenbeek emphasised the importance of considering these differences when interpreting the FVC decline.18
Efficacy and Safety of Nerandomilast in Patients with Idiopathic Pulmonary Fibrosis or Interstitial Lung Disease
Primary Endpoint: Change in Lung Function (Forced Vital Capacity) up to Week 52
In both FIBRONEER-ILD and FIBRONEER-IPF, the primary endpoint was achieved for both doses of nerandomilast (9 mg and 18 mg twice daily), reducing the decline in FVC compared to placebo.16,17 Richeldi and Maher noted that in both FIBRONEER-ILD and FIBRONEER-IPF, the FVC curves for nerandomilast (both doses) and placebo separated early and continued to diverge over the 52 weeks. Maher also stated that “there was a clear benefit in both dosing arms” across all groups. Data from FIBRONEER-ILD are shown in Figure 1. In FIBRONEER-ILD, the placebo group (n=391) had a mean decline of 165.8 mL, with relative reductions of 49% and 41% observed in the 9 mg (n=390; p<0.001) and 18 mg (n=390; p<0.001) nerandomilast groups, respectively (Figure 2A).16 In the FIBRONEER-IPF trial, the placebo group (n=391) had a mean FVC decline of 183.5 mL at Week 52. This was reduced by 24% in the nerandomilast 9 mg group (n=390; p=0.02) and by 38% in the 18 mg group (n=392; p<0.001; Figure 2B).17 Wijsenbeek highlighted that, when grouping all patients from the ILD or IPF trials, the placebo group was not entirely untreated as this includes patients concurrently taking background antifibrotic therapy.18
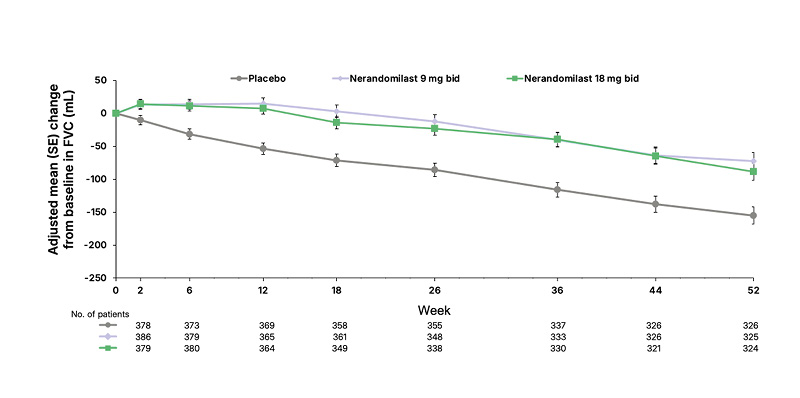
Figure 1: Change in forced vital capacity over 52 weeks in the FIBRONEER-ILD.
Adapted from Maher TM et al.16
©Boehringer Ingelheim International GmbH
BID: twice daily; FVC: forced vital capacity; SE: standard error.
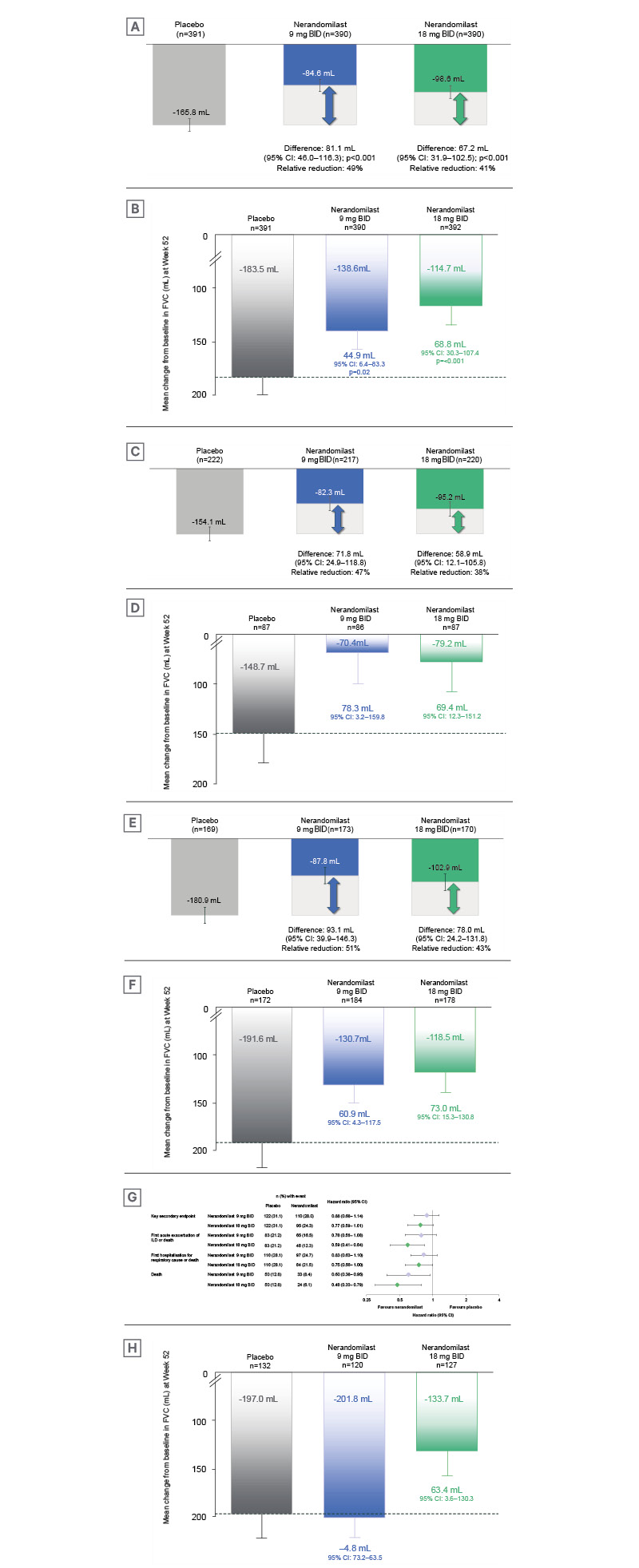
Figure 2: Change in forced vital capacity at Week 52 across the FIBRONEER-IPF and FIBRONEER-ILD trials.16,17
Adjusted mean (standard error) change in FVC (mL) from baseline to Week 52 is shown for:
A) All patients in the FIBRONEER-ILD study.16 B) All patients in the FIBRONEER-IPF study.17 C) FIBRONEER-ILD
patients not taking background nintedanib. D) FIBRONEER-IPF patients not taking background nintedanib or
pirfenidone. E) FIBRONEER-ILD patients taking background nintedanib. F) FIBRONEER-IPF patients taking background nintedanib. G) Forest plot and hazard ratios for the key secondary endpoint* and secondary time-to-event endpoints in FIBRONEER-ILD, up to first database lock (DBL1), comparing placebo and nerandomilast. H) FIBRONEER-IPF
patients taking background pirfenidone.
*Time to first acute exacerbation, hospitalisation for respiratory cause, or death. Mean exposure to trial medication was 14.4 months.
Adapted from Maher TM et al.16 and Richeldi L et al.17
©Boehringer Ingelheim International GmbH
BID: twice daily; FVC: forced vital capacity; ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis.
In FIBRONEER-ILD, 56.3% of patients were not taking background antifibrotics. Amongst the patients not taking background therapy, the placebo group (n=222) had a mean FVC decline of 154.1 mL, with reductions in FVC of 47% (n=217) and 38% (n=220) with the 9 mg and 18 mg doses, respectively (Figure 2C).16 In the FIBRONEER-IPF trial, 22.3% of patients were not taking background antifibrotic therapy. Among patients not taking background therapy in FIBRONEER-IPF (n=87), the relative decline in FVC was greater than that of patients on background antifibrotics. Placebo-treated individuals had a mean decline of 148.7 mL, compared with 53% and 47% relative reductions for nerandomilast 9 mg (n=86) and 18 mg (n=87), respectively (Figure 2D).17 Richeldi explained that the placebo group in patients not taking background antifibrotic therapy could be considered as a ‘true placebo group’. Richeldi emphasised awareness of this group when interpreting both primary and secondary endpoints.
In the subgroup taking background nintedanib, FVC decline at Week 52 in the placebo group was 180.9 mL in FIBRONEER-ILD (n=169), compared with 51% and 43% reductions in the 9 mg (n=173) and 18 mg (n=170) groups, respectively (Figure 2E).16 Similarly, in FIBRONEER-IPF, the placebo group (n=172) declined by 191.6 mL, with relative reductions of 32% for 9 mg (n=184) and 38% for 18 mg (n=178; Figure 2F).17
Among patients on background pirfenidone in FIBRONEER-IPF (Figure 2H), the placebo group (n=132) showed a mean FVC decline of 197.0 mL. Notably, only the nerandomilast 18 mg group (n=127) showed a relative reduction in FVC, at 32%, with no effect seen in the 9 mg group (n=120) compared to placebo.
Drug–Drug Interaction with Nerandomilast
Richeldi attributed the reduced efficacy of nerandomilast 9 mg in patients taking pirfenidone in FIBRONEER-IPF to a drug–drug interaction. Wijsenbeek reported that for those taking background pirfenidone, the nerandomilast plasma concentrations nearly halved (approximately 50%) over 26 weeks, compared to those taking nintedanib or with no background therapy.17 Wijsenbeek noted that excluding these patients revealed a 37–40% relative reduction in FVC decline with both nerandomilast doses. Further investigation into the underlying mechanism of action is needed.
Secondary Time-to-Event Endpoints
In FIBRONEER-ILD, a nominally significant reduction in risk of death was observed (hazard ratio [HR]: 0.60; 95% CI: 0.38–0.95; Figure 2G).16 Maher also reported that at DBL1, nerandomilast 9 mg twice daily showed a non-significant reduction in KSE risk (HR: 0.88; 95% CI: 0.68–1.14; p=0.34) compared with placebo.16 For the nerandomilast 18 mg group, the KSE risk was also nominally lower (HR: 0.77; 95% CI: 0.59–1.01; p=0.06),16 with a nominal reduction in risk of death (HR: 0.48; 95% CI: 0.30–0.79).16 Maher commented that, although results at DBL1 were non-significant, “I think unequivocally, we are seeing a benefit in terms of mortality in these patients.”
For FIBRONEER-IPF, neither dose of nerandomilast demonstrated an effect on KSEs at DBL1, with HRs near 1 for both doses (Figure 2H).17 Richeldi and Wijsenbeek highlighted the relevance of the DBL2 data, and the effect of increased follow-up time on results, with Wijsenbeek noting there was a 17% increase in the number of events for KSEs between DBL1 and DBL2, along with a 39% increase in deaths.18 At DBL2, Wijsenbeek noted a trend favouring nerandomilast 18 mg dose, with a reduction in the risk of deaths from 42 deaths in placebo to 26 deaths in the nerandomilast 18 mg group (HR: 0.66; 95% CI: 0.41–1.08) and a nominally significant reduction in risk of FVC decline predicted >10% or death (HR: 0.75; 95% CI: 0.59–0.95).17 Referring to the Kaplan-Meier curve of the KSE, Wijsenbeek reported that the difference in KSEs became more pronounced with increased exposure and a greater number of events. It was noted that there was no effect in patients taking background pirfenidone, again due to drug–drug interaction. Wijsenbeek also reported that a signal in favour of the KSE was observed in patients with no background therapy receiving nerandomilast.18
Safety and Tolerability
Wijsenbeek emphasised the importance of safety in current treatments for ILDs. Both nintedanib and pirfenidone exhibit a high burden of gastrointestinal side effects, including diarrhoea, nausea, and vomiting.19 In the INBUILD trial of nintedanib for patients with progressive fibrosing ILDs, diarrhoea was the most frequently reported adverse event (AE), with almost 50% of patients who were taking nintedanib requiring ≥1 dose reduction and/or treatment interruption, compared to 15% in the placebo group.20 Both FIBRONEER-ILD and FIBRONEER-IPF trials demonstrated that nerandomilast 9 mg and 18 mg twice daily had favourable safety and tolerability profiles across treatment groups.16,17 Serious AEs and severe AEs were balanced across all groups (Table 1).16,17 AEs of interest, such as psychiatric AEs (including depression and suicidal ideation), which Wijsenbeek noted have been reported with other PDE4 inhibitors, were equally distributed across treatment groups.16,17 Other AEs of interest, including infections, drug-induced liver injury, and vasculitis, were also balanced across the treatment groups (Table 1). In both trials, diarrhoea was the most common AE.16,18
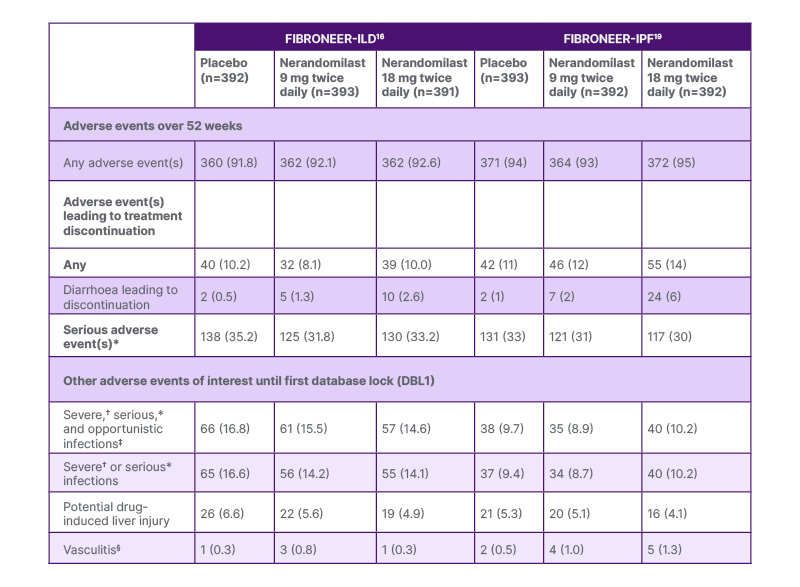
Table 1: Summary of safety and adverse events for all patients in FIBRONEER-ILD and FIBRONEER-IPF.16,17
Data are n (%) of patients with ≥1 adverse event reported over the 52-week treatment period or until 7 days after the last dose in patients who discontinued treatment before Week 52.
*An event that resulted in death, hospitalisation or prolongation of hospitalisation, or persistent or clinically significant disability or incapacity; or was life-threatening, a congenital anomaly or birth defect, or deemed to be serious for any other reason.
†Common Terminology Criteria for Adverse Events of Grade ≥3.
‡Including Mycobacterium tuberculosis infections.
§In FIBRONEER-IPF: three patients in the nerandomilast 18 mg twice daily group, one patient in the nerandomilast 9 mg twice daily group, and one patient in the placebo group had an on-treatment adverse event adjudicated as
vasculitis by an independent adjudication committee. In FIBRONEER-ILD: two patients in the nerandomilast 9 mg twice daily group, one patient in the nerandomilast 18 mg twice daily group, and one patient in the placebo group had an on-treatment adverse event adjudicated as vasculitis by an independent adjudication committee.
Adapted from Maher TM et al.16 and Richeldi L et al.17
©Boehringer Ingelheim International GmbH
ILD: interstitial lung disease; IPF: idiopathic pulmonary fibrosis.
In FIBRONEER-ILD, Maher reported discontinuation due to AEs in 10.2% of the placebo, 8.1% of the nerandomilast 9 mg, and 10.0% of the nerandomilast 18 mg group. The AEs leading to discontinuation over 52 weeks for patients not taking and taking background nintedanib (7.3–11.7%) were comparable to placebo (10.0–10.4%).16 Diarrhoea leading to treatment discontinuation was more frequent in those taking background nintedanib; however, overall, there were few discontinuations (<4%) due to diarrhoea (Table 1).16,17 In FIBRONEER-IPF, Wijsenbeek reported that discontinuation due to AEs occurred in 11% of the placebo, 12% of the nerandomilast 9 mg, and 14% of the nerandomilast 18 mg group.17 In patients who were not taking any background antifibrotics, discontinuations occurred in 8% of the placebo, and in 8% and 7% of the nerandomilast 9 mg and 18 mg groups, respectively. Among patients taking background nintedanib, discontinuations were more frequent (17% for nerandomilast 9 mg and 21% for nerandomilast 18 mg), compared with placebo (13%).17 In patients taking background pirfenidone, discontinuation due to AEs occurred in 7% and 9% of those receiving nerandomilast 9 mg and 18 mg, respectively, compared with placebo (10%), although Wijsenbeek noted that these data should be interpreted cautiously due to the reduced nerandomilast plasma concentrations in this subgroup.
Overall Learnings From FIBRONEER Trials
The Phase III FIBRONEER-ILD and FIBRONEER-IPF trials both met the primary endpoint, demonstrating that nerandomilast, at 9 mg and 18 mg twice daily, significantly reduced the decline in FVC over 52 weeks, with a favourable safety and tolerability profile, in patients with IPF and PPF, with or without background antifibrotic therapy.16,17
A numerical reduction in the risk of the KSE, time to first acute exacerbation, hospitalisation for respiratory cause, or death, over the duration of the trial, and a nominally significant reduction in the risk of death was observed in patients receiving nerandomilast compared to placebo.16,17 A key difference in FIBRONEER-IPF was that the use of pirfenidone was allowed as background therapy, which influenced results as a result of the drug-drug interaction with neradnomilast.17 Further, more trial data were available for FIBRONEER-IPF at the time of the presentation (DBL2), providing a more complete dataset with extended follow-up for time-to-event endpoints.
Richeldi highlighted that patients taking background antifibrotic therapy (nintedanib or pirfenidone) represent a more rapidly progressing population, and the greater decline in FVC seen in placebo with these subgroups indicates that these patients “had more severe disease at baseline,” illustrated by a greater decline in FVC compared to those not taking background therapy. For FIBRONEER-ILD, FVC decline was 180.9 mL in patients taking background nintedanib compared to those not taking background therapy (154.1 mL).16 Similarly, for FIBRONEER-IPF, FVC decline was reported as 191.6 mL for nintedanib and 197.0 mL for pirfenidone, compared to 148.7 mL for those not taking background therapy.17
These data showing significant decline in FVC despite taking nintedanib or pirfenidone (placebo groups) highlight the ongoing unmet need for additional therapeutic options, with Wijsenbeek reporting that this “shows the need for additional therapies, even for patients already taking an approved therapy.”
Of note, in the subgroup of patients not taking background therapy in FIBRONEER-IPF, the relative reduction in FVC was greater than that of patients on background antifibrotics.17 Richeldi highlighted the placebo group in patients not taking background AF therapy, citing them as a ‘true placebo group’, to be considered when interpreting both primary and secondary endpoints.16,17
Richeldi also emphasised the importance of sufficient follow-up time for evaluating time-to-event endpoints. In FIBRONEER-IPF, accelerated recruitment led to shorter-than-planned exposure to study medication, reducing the planned observation period.16,17
Taken together, the efficacy and safety underscore the potential of nerandomilast, particularly as a monotherapy, or in those patients not receiving background antifibrotic therapy. Richeldi described these trial findings as a major step forward in the treatment of IPF and highlighted the need for personalised treatment approaches in ILD.17
Delayed Initiation of Supplemental O2 with Nerandomilast
During FIBRONEER-ILD and FIBRONEER-IPF, 27.6% and 21.1% of patients used supplemental O2 at baseline, respectively.16,17 Initiation of supplemental O2 can impact patients with ILD psychologically.21
In a late-breaker presentation,22 Oldham reported on the time to initiation of supplemental O2 in patients from the FIBRONEER-IPF study who were not receiving supplemental O2 at baseline (as determined by the Living with Pulmonary Fibrosis [L-PF] questionnaire).23 Nerandomilast 9 mg twice daily reduced the risk of time to O2 initiation (HR: 0.67; 95% CI: 0.46–0.98), whereas this effect was not observed with the 18 mg twice daily dose (HR: 0.92; 95% CI: 0.64–1.32).22 However, until Month 12, the data for both doses were almost the same.22
Oldham reported that “the effect of nerandomilast on reducing the risk of initiating O2 was seen mainly in the subgroup of patients with a baseline DLCO <50% predicted.” A post-hoc analysis showed an HR of 0.52 (95% CI: 0.31–0.87) for nerandomilast 9 mg, and 0.93 (95% CI: 0.60–1.46) for 18 mg, compared to placebo.22
For patients with a better preserved DLCO (DLCO ≥50% predicted), an HR of 0.97 (95% CI: 0.46–2.05) for nerandomilast 9 mg and 0.92 (95% CI: 0.42–1.99) for 18 mg was seen compared with placebo.22 Changes in supplemental O2 flow rate (at rest, when sleeping, or with exertion) were inconclusive due to limited data.22
Impact On Clinical Management and Patient Care with Nerandomilast
Oldham concluded that nerandomilast may delay supplemental O2 initiation in patients with IPF, particularly those with more severe disease (DLCO <50% predicted), potentially improving patient quality of life. Further research is needed to confirm these findings and evaluate the long-term benefits of nerandomilast in different patient subgroups.
Mechanisms of Action and Drug Discovery Strategies for Nerandomilast in Idiopathic Pulmonary Fibrosis and Progressive Pulmonary Fibrosis
Current IPF treatments primarily target the growth or migration of scar-forming fibroblasts;6,24 however, novel therapies like nerandomilast may be more effective by targeting multiple cell types, in addition to fibroblasts, and pathways involved in IPF and PPF pathobiology.14,15,24-26 Thomas presented a poster highlighting that the efficacy of nerandomilast in IPF and PPF6,16,17 may be attributable to a multifaceted mechanism of action on an array of antifibrotic and immunomodulatory effects,6 such as epithelial function,15,25 endothelial vascular function,25,26 and immune function,14 in addition to fibroblast function.14,25
Nerandomilast Mechanisms of Action
Thomas highlighted that the effects of nerandomilast were linked to activated cAMP-associated pathways, the modulation of G-protein-coupled receptor signalling, MAPK signalling pathways, and transforming growth factor β 1 signalling, key pathways in fibroblast function.6,25 Using in vitro cytokine-stimulated airway epithelial cell cultures, and in vivo adeno-associated virus-targeted diphtheria toxin murine model of acute lung injury, nerandomilast demonstrated stabilising endothelial barrier integrity by reducing microvascular permeability, epithelial damage, and epithelial cell activation.6
Nerandomilast also suppressed inflammatory activation of endothelial cells and innate immune cell adhesion and infiltration, inhibiting cytokine release from peripheral blood mononuclear cells, macrophages, and activated T cells in vitro.6
Nerandomilast demonstrated anti-fibrotic properties by inhibiting fibroblast proliferation, significantly reducing the release of biomarkers of fibrogenesis from the epithelial cell cultures.6 Myofibroblast contractility was also inhibited, accompanied by markers of de-differentiation towards a more normal fibroblast.6
Novel Analytics Using AI for Next-Generation Targeting to Transform Drug Discovery Strategies
Thomas also highlighted future drug target discovery strategies for IPF and PPF using spatial multi-omic analysis, AI-assisted systems, including a gene prioritisation algorithm and large language models, and advanced in vitro co-culture and organoid test systems.6 These approaches aim to identify and validate targets aligned with specific cell types within disease-associated niches, using AI-assisted target finding.6 Thomas concluded that these next-generation targets could not only limit disease but also restore functionality to the lungs.
Pharmacokinetics and Drug–Drug Interactions of Nerandomilast with CYP3A Inhibitors
Nerandomilast is primarily metabolised by cytochrome P450 3A (CYP3A), a major drug-metabolising enzyme.27 Several posters presented at ATS 2025 examined the in vivo pharmacokinetics (PK) and potential drug–drug interactions of nerandomilast, including co-administration with itraconazole,26 a strong CYP3A inhibitor,4,28-30 indicating that CYP3A inhibition may result in increased nerandomilast exposure, and therefore, potentially necessitates dose adjustments.27 A previous study showed that nerandomilast was not considered to be a CYP3A inducer, and is therefore unlikely to impact the systemic exposure of drugs primarily metabolised by CYP3A and CYP2C enzymes that are induced via activation of the same pathway.31
In an open-label, fixed-sequence study, 16 healthy male participants (mean age: 42.3±10.2 years and BMI: 25.7±2.8 kg/m2) received a single oral dose of 6 mg nerandomilast alone and in combination with once daily 200 mg itraconazole.27
Yu summarised that co-administration with itraconazole increased nerandomilast area under the plasma concentration–time curve in plasma from time zero to 119 hours (AUC0₋119) by approximately 2.2 times (90% CI of gMean ratio: 203.5–242.5), area under the curve (AUC) time zero to infinity (AUC0-∞) by approximately 2.3 times, and maximum measured concentration in plasma (Cmax) by 1.3 times (90% CI of gMean ratio: 117.7–139.2) compared to nerandomilast monotherapy in healthy participants.27 Yu reported that itraconazole delayed peak plasma concentration of nerandomilast by approximately 1 hour, while the decrease in plasma concentration in the terminal phase was similar.
Nerandomilast, both as a monotherapy and co-administered with itraconazole, had an acceptable safety profile in healthy participants. All AEs were of mild or moderate intensity, no AEs led to treatment discontinuation, and no severe AEs, serious AEs, or other significant AEs were reported.27
Yu summarised that CYP3A inhibitors increase nerandomilast exposure, implying that clinical caution, and evaluation of potential drug–drug interactions and dosing adjustments to maintain safety and efficacy, are warranted.
Nerandomilast in Participants With and Without Renal Impairment
Renal impairment (RI) is common in IPF; in a previous Phase II trial examining nerandomilast, 76% of participants had mild or moderate RI.32 A poster presented by Choi evaluated the PK of a single 18 mg dose of nerandomilast in participants with moderate (estimated glomerular filtration rate [eGFR]: 30–59 mL/min/1.73m2; n=8) or severe RI (eGFR: 15–29 mL/min/1.73m2; n=8) compared to individual-matched controls (based on age, sex, and weight) with normal renal function (eGFR ≥90 mL/min/1.73m2; n=10, with 6 matched to both RI groups).33
This non-randomised, open-label, parallel trial (n=26; mean age: 63.8±11.6 years, weight 73.5±12.7 kg with a mean [SD] BMI of 25.6 [3.6] kg/m2) showed that Cmax was slightly decreased in RI (3% in moderate, 14% in severe) while overall exposure (AUC from time zero to the last quantifiable concentration [AUC0-tz]) was increased by 37% for moderate RI, and 29% for severe RI, compared with matched controls with normal renal function.33 Plasma concentration–time profiles were similar across groups, indicating consistent drug behaviour despite RI.33 Renal clearance decreased with increasing RI severity, with urinary excretion (the fraction of nerandomilast excreted in urine) reduced from 13% (matched controls) to 9% in moderate RI and 6% in severe RI, suggesting altered clearance mechanisms in RI.33
Nerandomilast demonstrated an acceptable safety and tolerability profile, with mild-to-moderate treatment-emergent AEs occurring in 35% of participants across all groups, all of which resolved by the end of the observation period.33
Choi summarised that no dose adjustment is required for nerandomilast in patients with mild, moderate, or severe RI. However, use in end-stage renal disease (eGFR <15 mL/min/1.73m2) is not recommended as the PK, safety, and efficacy have not been investigated.33
Nerandomilast in Participants With and Without Hepatic Impairment
Hepatic impairment (HI) may alter CYP3A activity, the main enzyme responsible for metabolising nerandomilast.34,35 A poster presented by Madari evaluated the PK of a single 18 mg dose of nerandomilast in participants with mild (Child-Pugh A [score: 5–6 points]; n=8) or moderate HI (Child-Pugh B [score: 7–9 points]; n=8) compared to individual-matched controls (based on age [±10 years], sex, and weight [±15%]) with normal hepatic function (n=12; four matched to both HI groups).35
This non-randomised, open-label, parallel trial, (n=28; mean age: 64.0±7.7 years; BMI: 27.3±4.2 kg/m2) showed that Cmax decreased in HI (by 17% in mild, and 31% in moderate), while overall exposure (AUC0-tz and AUC0-∞) increased by 5% for mild HI and 31% for moderate HI compared with matched controls with normal hepatic function.35 Plasma concentration–time profiles showed greater differences in moderate HI compared to controls than in mild HI, indicating a dose-dependent effect of increasing HI severity.35
Nerandomilast showed an acceptable tolerability in participants with or without mild or moderate HI. Madari reported that one participant with mild HI experienced a moderate AE of subcutaneous haematoma.35 Six participants (21%) reported at least one treatment-emergent AE, all of mild intensity, which resolved by the end of the trial.35
Madari summarised that no dose adjustment is required in patients with mild or moderate HI. However, use of nerandomilast in patients with severe HI (Child-Pugh C) is not recommended, and further research is needed.
Summary
The Phase III FIBRONEER trials, FIBRONEER-ILD and FIBRONEER-IPF, demonstrated that nerandomilast slows down disease progression in IPF and PPF, with the greatest benefit observed in those patients not receiving background antifibrotic therapy.16,17
Nerandomilast exhibited an acceptable safety profile, with a numerical reduction in the risk of death.16,17 Nerandomilast was also found to delay the initiation of supplemental O2 in patients with IPF. PK data showed that while nerandomilast is metabolised by the CYP3A enzyme, it is not a CYP3A inducer.27 Finally, nerandomilast can be used in patients with mild or moderate renal and hepatic impairment without the need for dose adjustment.33,35







