BACKGROUND AND AIMS
The vaccine against herpes zoster (HZ), known as the recombinant zoster vaccine (RZV) is recommended by the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) for individuals who are immunocompromised. Short-term observational data from rheumatoid arthritis (RA) cohorts aged ≥50 years support its overall reactogenicity and possible safety in non-randomized designs.1-3 However, the specific impact of different immunosuppressive drugs or combinations on vaccine immunogenicity are limited to small sample sizes, hampering conclusions about the effect of specific therapies. To address these gaps, the authors conducted a large prospective RCT to evaluate safety and assess humoral and cellular immunogenicity induced by RZV in adult (≥18 years) patients with RA.4,5
MATERIALS AND METHODS
Patients were randomized to receive RZV (P1) or placebo (P2) at Day (D)0 and D42. Groups were blindly evaluated for disease activity (Clinical Disease Activity Index [CDAI], Simple Disease Activity Index [SDAI], and Disease Activity Score-28 for Rheumatoid Arthritis with CRP [DAS28-CRP]) at D0, D42, and D84 and classified as worsening disease activity at D84 according to the respective worsening disease activity criteria and the patient perception. The P2 group was vaccinated with a similar schedule at D84 and D126. Anti-gE antibody concentrations (ELISA) were assessed before first RZV dose and 6 weeks after the second dose. Pre- and post-vaccination geometric mean titers (GMT) were calculated. Cellular immunogenicity was measured at baseline and 6 weeks after full vaccination by cytometry after in vitro stimulation with gE ectodomain: frequency and number of gE-specific CD4+[2+] T cells producing at least 2/4 markers (IFN-γ, IL-2, TNF-α, and CD40 ligand) were measured in a sample of patients and controls. Adverse events were monitored for 3 months through scheduled visits and phone contacts.
RESULTS
A total of 320 patients with RA were randomized at D0: 165 in P1 and 155 in P2 (Figure 1). The groups were well balanced for age (p=0.562), sex (p=0.331), previous HZ infections (p=0.580), disease duration (p=0.838), rheumatoid factor (p=0.411), antic-cyclic citrullinated peptide antibody (p=0.456) positivity, disease modifying anti-rheumatic drugs (DMARD) distribution (p>0.05), DAS28-CPR (p=0.268), and SDAI (p=0.343). After two vaccine doses, seroconversion (96.6% versus 100%; p=0.030) and GMT (8.3 [7.2-9.5] versus 12.9 [11.5-14.5 mUI/mL]; p<0.001) were significantly reduced in patients compared to control group. No factors were associated with absence of SC (p>0.05). Linear regression corrected for pre-vaccination GMT linked older age (p=0.032), higher disease activity (p=0.042), methotrexate (MTX; p>0.001), and prednisone use (p=0.045) with lower post vaccination GMT. The concomitant use of MTX reduced post-vaccination GMT among biologic DMARD (p<0.001) users (mainly TNF inhibitor users; p=0.003) and synthetic DMARD users (p<0.001). In multivariate analysis, higher pre-vaccination GMT persisted, associated with higher post-vaccination GMT (<0.001), while older age (>60 years; p=0.033) and MTX use (p<0.001) remained associated with lower post-vaccination GMT (effect estimate ratio [95% confidence interval]: 0.761 [0.590-0.981] and 0.562 [0.399–0.791], respectively). Cellular immunogenicity was measured in 42 patients with RA and 29 controls, who showed comparable responses according to the frequency (p=0.233) and number (p=0.531) of specific CD4[2+] T cells. No HZ cases occurred by Week 12. The frequencies of patients worsening disease activity were comparable between P1 (vaccine) and P2 (placebo) at D42 and D84 according to delta CDAI, delta SDAI, and DAS28-CRP worsening criteria (p>0.05 for all). No moderate/severe adverse events were observed.
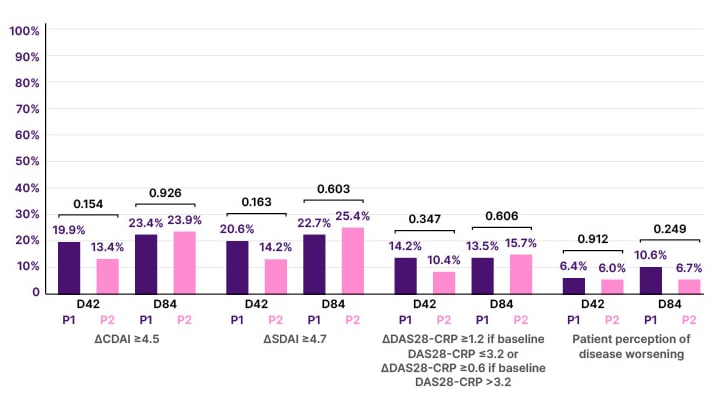
Figure 1: Frequencies of worsening rheumatoid arthritis disease activity at Day 42 and Day 84, 6 weeks after the first and second doses of recombinant zoster vaccine (P1) or placebo (P2).
CDAI: Clinical Disease Activity Index; CRP: C-reactive protein; DAS28-CRP: Disease Activity Score-28 for Rheumatoid Arthritis with CRP; SDAI: Simple Disease Activity Index;.
CONCLUSION
This large study showed that RVZ was safe in immunosuppressed patients with RA, with no evidence of increased flare rate using validated measures. Importantly, the authors identified MTX and older age as key factors that significantly impair vaccine-induced immune response. These findings emphasize the need for drug discontinuation strategies or booster doses to optimize long-term protection.







