BACKGROUND AND AIMS
Immune checkpoint inhibitors (ICI) are efficacious treatments for various cancers. As approvals for ICI treatment increase for additional cancers, the prevalence of rheumatologic immune related adverse events (irAE) also grows. First-line treatment for these irAEs is glucocorticoids; however, there is a lack of standardization in dosing, tapering, and duration of treatment. There are varying results published on ICI-treated patients on the association of oral glucocorticoids on progression-free survival (PFS).1
MATERIALS AND METHODS
Data from a prospective USA multi-center rheumatic irAE cohort (RADIOS) were utilized. Inclusion criteria for this study were the following: patients enrolled since February 2023; treated with an ICI for cancer; diagnosed with ICI-inflammatory arthritis (ICI-IA), defined as inflammatory arthritis, arthralgia, or polymyalgia rheumatica; and treated with glucocorticoids. Patients with pre-existing autoimmune disease, or treatment for another irAE with glucocorticoids, were excluded. Data on demographics, cancer and cancer treatment, disease-modifying anti-rheumatic drugs, and glucocorticoid treatment were collected. Glucocorticoid dosage was converted to prednisone equivalents. Cumulative glucocorticoid exposure and the average daily prednisone dose was calculated at different time points and displayed in a box plot. Descriptive statistics were performed. Thirty-day landmark Kaplan-Meier plots were drawn to investigate glucocorticoid treatment and cancer progression using time from glucocorticoid initiation to radiographic cancer progression or death (PFS). Time-varying Cox proportional hazard models were also performed using time from ICI initiation to glucocorticoid treatment. Adjusted models included the following covariates: age, irAE grade at baseline, cancer type (melanoma, non-small cell lung cancer, renal cell cancer), cancer stage, and ICI combination therapy.
RESULTS
The analytic cohort consisted of 206 patients with a mean age of 65 years (SD: 12.36), 48.5% were female, and 85.9% were White (Table 1). The most frequent cancers were melanoma (32.5%), renal cell cancer (18.4%), or non-small cell lung cancer (11.7%), and were Stage 3 (26.7%) or 4 (58.3%). Time from ICI-IA diagnosis to glucocorticoid initiation was a median of 24.5 days (interquartile range: 3–63). Median time from glucocorticoid initiation to cancer progression was 82.5 days (interquartile range: 70–283) among the 46 patients (22.3%) who progressed. In a landmark Kaplan-Meier curve the median glucocorticoid dose in the first month of treatment was not associated with PFS (log-rank p value 0.99). Similarly, using quartiles of glucocorticoid dose, there was also no association between glucocorticoid use and PFS (log rank p value 0.31). Lastly, all of the adjusted Cox models were not significant.
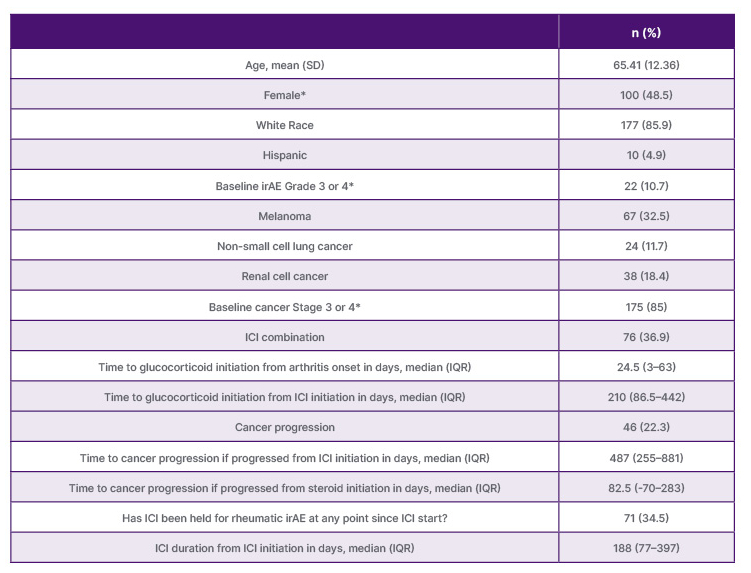
Table 1: Demographic and cancer characteristics (N=206).
*Missing data: N=1 for female; N=40 for baseline irAE grade; N=17 baseline cancer stage.
ICI: immune checkpoint inhibitor; IQR: interquartile range; irAE: immune related adverse events.
CONCLUSION
Using data from RADIOS, the authors found no association between glucocorticoid treatment and PFS in patients with ICI-IA. Rheumatologists often prescribe glucocorticoids at lower doses than oncology guidelines recommend. These findings suggest that glucocorticoid treatment by rheumatologists for ICI-IA may not have a substantial impact on cancer outcomes.







