BACKGROUND
Multiparametric MRI (mpMRI) with contrast medium is recommended in the prostate cancer diagnostic pathway.1,2 It is unclear if MRI without contrast medium (biparametric [bp]) can be used instead whilst remaining sensitive to the detection of clinically significant cancers.3 Additionally, for those with a positive MRI, is image-fusion targeting better than visual-registration (cognitive) targeting in detecting clinically significant prostate cancer?4 And does bpMRI represent better value for money than mpMRI? A randomised controlled trial testing the clinical utility and cost-effectiveness of these approaches is vital before changes in practice.
METHODS
IP7-PACIFIC is a prospective, multicentre, co-enrolment trial with two randomisations and embedded economic evaluation (Figure 1). The first randomisation will evaluate non-inferiority of bpMRI compared to mpMRI in those with clinical suspicion of prostate cancer. Men with a suspicious MRI will undergo a second randomisation to evaluate if image-fusion targeting is superior to standard visual-registration targeted biopsy. Ethics committee approval has been granted by the London Bromley Research Ethics Committee.
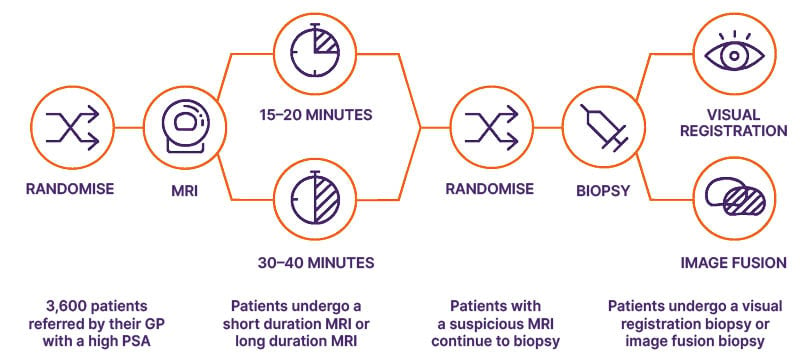
Figure 1: Study schema.
GP: general practitioner; PSA: prostate-specific antigen.
RESULTS
The primary objective for Randomisation 1 is to determine the non-inferiority of bpMRI to detect Gleason score ≥7 cancer (International Society of Urological Pathology Grade Group [GG] ≥2) compared to mpMRI. The objective for Randomisation 2 is to determine if image-fusion targeted biopsy is superior to visual-registration targeted biopsy for GG ≥2 cancer detection. An internal pilot phase will enrol 700 patients; the overall recruitment target is 2,600–3,600 pending interim analysis.
DISCUSSION
IP7-PACIFIC aims to provide randomised comparative evidence for the clinical utility and cost-effectiveness of using bpMRI and image-fusion biopsy. The findings will inform guidelines. The sequential randomised co-enrolment design allows simultaneous evaluation of two research questions and avoids heterogeneity of trial populations. By contrast to previous paired-cohort studies, the randomised design will reduce reporter bias, providing the highest level of diagnostic evidence.