Abstract
Background and Aim: The prevalence of depressive symptoms and major depressive disorder is high among adults living with HIV. Depressive symptoms are associated with increased cardiovascular disease risk. This study examined the association between depressive symptoms and echocardiographic indices of left ventricular diastolic dysfunction (LVDD) among men and women living with and without HIV.
Methods: Cross-sectional analysis included individuals in the Multicenter AIDS Cohort Study (MACS) and Women’s Interagency HIV Study (WIHS) who participated in transthoracic echocardiogram substudies and completed measures of depressive symptoms at the same visit as, or up to 6 months prior to, the transthoracic echocardiogram visit. Participants had helper T cells (CD4) >350 cells/mm3 and HIV RNA viral load <499 copies/mL. The presence of LVDD was defined according to the Characterizing Heart Function on Antiretroviral Therapy (CHART) criteria. Secondary outcomes were continuous values of each component of the CHART criteria: left ventricular ejection fraction >50%, septal e’ velocity, lateral e’ velocity, left atrial volume index, left ventricular mass index, and relative wall thickness. Logistic and linear regression were used to adjust for sociodemographic, behavioural, cardiometabolic, and HIV-related factors.
Results: Among 874 men (51% with HIV) and 1,191 women (76% with HIV), in whom the overall prevalence of LVDD was 22.5% and depressive symptoms 30.8%, depressive symptoms were not significantly associated with LVDD. The associations between individual LVDD components and depression were in the small to medium range, though generally not significant.
Conclusion: Findings warrant further research regarding the association between LVDD and depressive symptoms in the era of combination antiretroviral therapy.
Key Points
1. Major depressive disorder and cardiovascular disease are common in patients living with HIV, yet the association between mental health and heart structure changes is not well known.2. This study examined the relationships between symptoms of depression and diastolic dysfunction by echocardiogram in two well-characterised, longstanding prospective cohorts of men and women living with and without HIV in the USA.
3. Depressive symptoms differed by gender, and when compared by HIV status, men living with HIV had more depressive symptoms. However, depressive symptoms were not associated with diastolic dysfunction in this study.
INTRODUCTION
The prevalence of both major depressive disorder and cardiovascular disease (CVD) is higher among persons living with HIV (PWH) when compared to the general population.1,2 Evidence suggests that both depression and HIV are associated with increased risk for CVD. Studies in the Women’s Interagency HIV Study (WIHS) and Multicenter AIDS Cohort Study (MACS) indicate that among PWH, 47% of women and 26% of men reported high levels of depressive symptoms,3-6 as well as high rates of heart failure.7,8 Depressive symptoms and major depressive disorder (MDD) can potentially increase the risk for CVD in the context of HIV, as both HIV and depression have been independently associated with CVD including heart failure (HF). 9 MDD has been associated with a 30% increase in the risk of myocardial infarction (MI) among PWH after adjusting for traditional CVD risk factors.10 It is unclear whether depressive symptoms impact CVD risk through the intensity and duration of depression.
While previous studies have documented some associations between HIV and changes in heart structure and function, including heart failure among men and women living with HIV, 11-13 and in relation to antiretroviral medications,13 there is limited data examining heart failure precursor specific knowledge among PWH in relation to depressive symptoms, which limits HIV-specific insights into CVD prevention and treatment.12,15 To address this gap, this study utilised data from two large prospective, multicentre cohort studies of men and women with and without HIV infection in the USA, the MACS and the WIHS, to investigate cross-sectional relationships between symptoms of depression and left ventricular diastolic disfunction (LVDD). In addition, this study evaluated the potential modification of these relationships by HIV status and sex, and associations between LVDD and depression among those living with HIV.
METHODS
Study Participants
This study used data collected from substudies conducted within two longstanding prospective cohort studies of the natural and treated histories of HIV-1 infection: the MACS and the WIHS (subsequently combined into the MACS/WIHS Combined Cohort Study [MWCCS] in 2019). The MACS was a multicenter study, established in 1984, among men with or at risk for HIV infection, conducted at four sites within the USA: Baltimore, Maryland/Washington, D.C.; Chicago, Illinois; Pittsburgh, Pennsylvania/Columbus, Ohio; and Los Angeles, California. 16,17 The WIHS was a multicentre study of women living with and without HIV that collected data from 10 sites throughout the USA starting in 1994: Bronx, New York; Brooklyn, New York; Washington, D.C.; Chicago, Illinois; San Francisco, California; Chapel Hill, North Carolina; Miami, Florida; Birmingham, Alabama; Jackson, Mississippi; and Atlanta, Georgia.18 Data collection instruments for each are available online on the MWCCS website.19 All sites obtained approval from their institutional review boards, and all participants provided written informed consent.
Data for the present study were obtained from transthoracic echocardiogram (TTE) sub-studies conducted within each cohort. Briefly, 1,195 participants in the MACS completed a TTE from 2017–2019, and 1,654 participants in the WIHS completed a TTE visit from 2014–2019.20 Details of the echocardiographic assessments have been described previously.12,20 Both studies included sonographer training and certification, standardised imaging protocols, and centralised imaging interpretation. Data available by 1 August 2022 were used in this analysis.
Participants were included in the present study if they completed a depression questionnaire at the same visit as, or up to 6 months prior to, the TTE visit. They were excluded if they were pregnant (due to physiologic changes during pregnancy), had HIV RNA viral load (VL) >499 copies/mL, a CD4+ T lymphocytes (CD4) cell count <350 cells/mm3 closest to the TTE visit, a personal or family history of CVD up to 2.5 years prior to the TTE visit, or systolic dysfunction (defined as having left ventricular ejection fraction <50%) at the TTE, or if diastolic dysfunction could not be calculated because of at least one key missing measurement in the component variables. Finally, they were excluded if they had missing data in any one of the key covariates in the main analysis, resulting in a reduction of 3.6% of the total sample after all other exclusions were applied (Supplementary Figure 1).
Measurement of Depressive Symptoms
Depressive symptoms were assessed using Center for Epidemiologic Studies Depression Scale (CES-D) scores and self-reported antidepressant medication use, assessed on the same visit as, or up to 6 months prior to, the TTE visit.21 The CES-D questionnaire contains 20 items on the frequency of self-reported depression symptoms, scored on a scale of 0 (rarely or none of the time) to 3 (most of the time). The final scale ranges from 0– 60, with higher scores indicating greater severity of depressive symptoms. A threshold of 16 was considered suggestive of depressive symptomatology, with the presence of depressive symptoms defined as either a CES-D score of ≥16 or self-reported use of antidepressant medication. A score of >20 was also considered to improve sensitivity and specificity in the context of HIV infection.22
Outcomes of Interest
The primary outcome of interest was the presence of LVDD, which was calculated using the criteria defined by the Characterizing Heart Function on Antiretroviral Therapy (CHART) Study.9,11 These criteria include: left ventricular (LV) ejection fraction ≥50%; evidence of impaired LV relaxation, defined as having either septal e’ velocity <7 cm/s or lateral e’ velocity <10 cm/s; evidence of chronically elevated LV filling pressure, defined as having left atrial volume index >28 mL/m2; LV hypertrophy, defined as having LV mass index >95g/m2 in women or >115 g/m2 in men; or concentric LV remodelling, defined as having relative wall thickness >0.42. Secondary outcomes of interest were the continuous values of each individual component used in the CHART criteria.9,11
Statistical Analyses
Descriptive analyses were conducted to assess the distributions (frequencies and proportions) of characteristics on or at the visit closest to the TTE visit, stratified by HIV serostatus and cohort. Regression analyses were used to evaluate the relationships between depressive symptoms with the presence of LVDD (logistic regression) and its individual components (linear regression). All logistic and linear regression models were run separately by cohort, which stratifies the analyses by sex assigned at birth and accounts for potential differences in the study populations and outcome ascertainment. For all outcomes, separately nested models were developed to serially adjust for sociodemographic, behavioural, traditional CVD, and HIV-related risk factors. Model 1 accounted for age (modelled as a natural spline term with two knots), self-reported race/ethnicity (White, Black, other), study site, smoking status (never, former, current), alcohol consumption (none, <14 drinks/week, ≥14 drinks/week), use of recreational drugs (poppers, crack, cocaine, uppers, injectable drugs, or other) since last visit, and HIV serostatus. Model 2 accounted for all variables in Model 1 as well as traditional CVD risk factors: BMI (weight [kg]/height [m]2, modelled as a natural spline term with two knots), hypertension (categorised as no hypertension [<140/90 mmHg] and no use of antihypertensive medications, controlled [<140/90 mmHg] and use of antihypertensive medication, or uncontrolled with or without medication [>140/90 mmHg]), diabetes (defined as present if ever self-reported anti-diabetic medication, fasting glucose ≥126 mg/dL, HbA1C≥6.5%, or self-reported diabetes [WIHS]; or current HbA1C ≥6.5%, fasting glucose ≥126 mg/dL, or diagnosed with diabetes and self-reported use of medications [MACS]), and treated or untreated hepatitis C virus (HCV) status at visit. Model 3 restricted each sample to those living with HIV, and further adjusted for undetectable viral load (defined as below the ratio of detection of the HIV RNA test used, which was 20 copies/mL), current CD4 cell count, nadir CD4 cell count, current use of cART, and history of clinical AIDS. Interaction terms assessed whether associations between depressive symptoms and LVDD and the continuous components of LVDD varied by HIV serostatus and cohort.
Since the prevalence of LVDD in the sample is relatively high (approximately 24%), modified Poisson regression with robust variance estimation was conducted in sensitivity analyses to assess potential overestimation of odds ratios (OR). Statistical significance was determined by a two-sided p<0.05. Analyses were conducted using Stata 17 (StataCorp, College Station, Texas, USA).
RESULTS
Descriptive Statistics
This study was comprised of 874 men (n=433 living with HIV) and 1,191 women (n=783 living with HIV), an average 54 years old (Table 1 and 2). One-third (32.6%) identified as White, 49.2% as Black, and 18.2% as other. One-third (33.1%) of participants were never smokers, whereas 30.3% were current smokers. Women were more likely to smoke compared to men (37.1% versus 21.1%). More than one-third (38.4%) reported no alcohol use since their last visit; alcohol use, defined as <14 drinks a week, was more frequent in women than in men (51.0% versus 21.1%). Overall, 18.3% had prevalent diabetes mellitus, including 13.8% of those in the MACS and 21.5% in the WIHS. Women were more likely to have HCV at the study visit compared to men (16.6% versus 4.9%). Men were more likely to report recreational drug use than women (30.5% versus 26.6%). Recreational drug use was higher among women living without HIV compared to those living with HIV (33.6% versus 23.0%). There were no notable differences by HIV serostatus or by cohort in the prevalence of hypertension. Men were more likely to have depressive symptoms than women (34.9% versus 27.9%). Among men, symptoms were higher among those living with HIV compared to those without HIV (39.7% versus 30.2%), but not in women (WIHS).
Adjusted Associations of Depressive Symptoms with Left Ventricular Diastolic Dysfunction and its Components
After adjustment for sociodemographic characteristics and CVD risk factors, depressive symptoms were not statistically significantly associated with LVDD in the MACS (OR: 0.94; 95% CI: 0.64, 1.37) or the WIHS (OR: 0.83; 95% CI: 0.57, 1.17) (Table 1 and 2). Associations between depressive symptoms and each of the individual components of LVDD by cohort and HIV serostatus were not statistically significant (Table 1 and 2). Among the MACS, the presence of depressive symptoms was associated with greater LV mass (β: 1.94, p=0.30) among men living with HIV and with lower LV mass (β: -3.8, p=0.06) among men not living with HIV. Despite these contrasting trends, the interaction by HIV was not statistically significant. Despite these overall differences, the interactions between HIV serostatus and cohort were not statistically significant. Poisson models showed a similar pattern of results.
Among participants living with HIV, associations between depressive symptoms and LVDD showed a similar pattern. Among both men and women living with HIV, depressive symptoms were associated with greater LV mass compared to those without depressive symptoms (β: 2.16 [95% CI:-1.51, 5.84] and 1.68 [95% CI: -0.77, 4.14]), although neither reached statistical significance at the 0.05 level. Using a cutoff of 20 on the CES-D scale instead of 16 resulted in similar findings for those living with HIV.
Among PWH, HIV clinical characteristics and LVDD were not statistically significantly associated except for nadir CD4+ T cell count among women (Table 3), though this was a small positive effect.
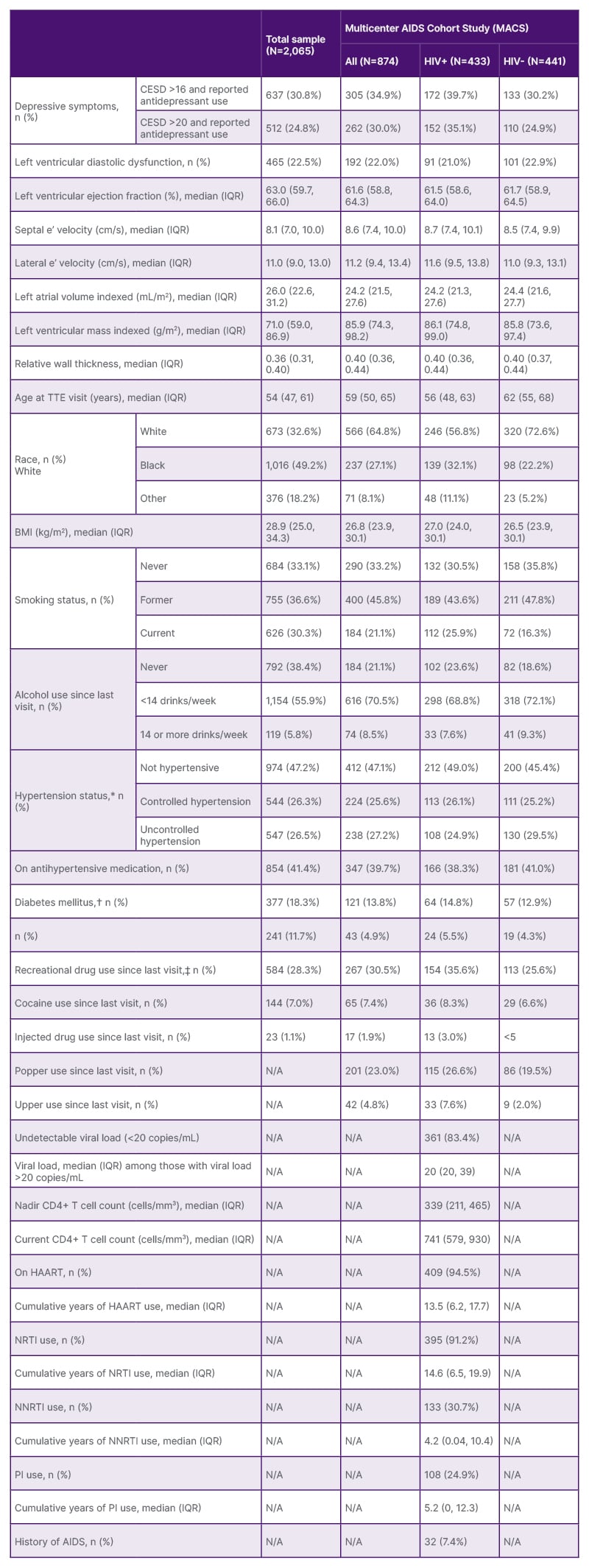
Table 1: Study population characteristics, by cohort and HIV serostatus.
*Uncontrolled hypertension defined as having a systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg. Controlled hypertension defined as having a systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg and taking anti-hypertensive medication. No hypertension defined as having a systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg and not taking anti-hypertensive medication.
†For MACS, diabetes mellitus defined as glycosylated haemoglobin ≥6.5% or fasting blood glucose ≥126 mg/dL (or diagnosed with diabetes and use of diabetes mellitus medications).
‡Poppers and uppers specified as separate drugs in MACS, while in WIHS, this is included with other drug use.
CD4: cluster of differentiation 4; CESD: Center for Epidemiologic Studies – Depression scale; HAART: highly active antiretroviral therapy; HIV+: HIV positive; HIV-: HIV negative; IQR: interquartile range; MACS: Multicenter AIDS Cohort Study; n (%): number of individuals and percentage of sample; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; TTE: transthoracic echocardiogram; WIHS: Women’s Interagency HIV Study.
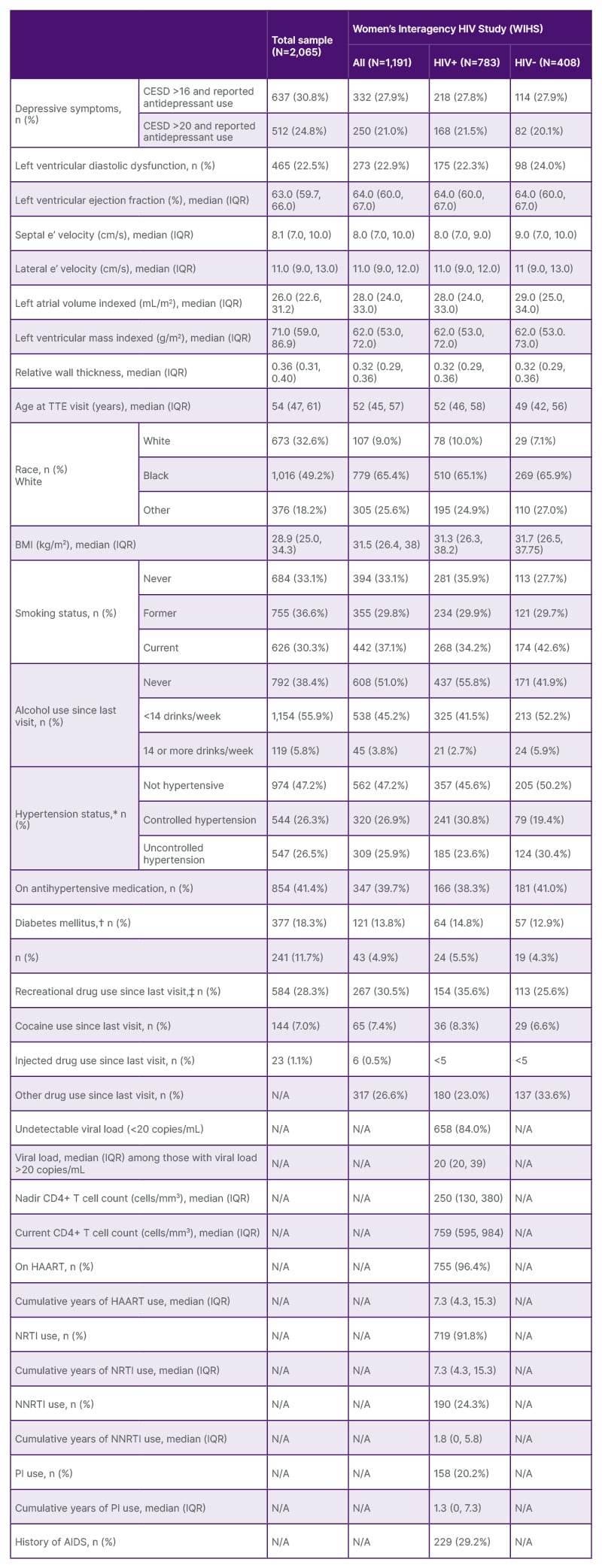
Table 2: Study population characteristics, by cohort and HIV serostatus.
*Uncontrolled hypertension defined as having a systolic blood pressure >140 mmHg or diastolic blood pressure >90 mmHg. Controlled hypertension defined as having a systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg and taking anti-hypertensive medication. No hypertension defined as having a systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg and not taking anti-hypertensive medication.
†For MACS, diabetes mellitus defined as glycosylated haemoglobin ≥6.5% or fasting blood glucose ≥126 mg/dL (or diagnosed with diabetes and use of diabetes mellitus medications).
‡Poppers and uppers specified as separate drugs in MACS, while in WIHS, this is included with other drug use.
CD4: cluster of differentiation 4; CESD: Center for Epidemiologic Studies – Depression scale; HAART: highly active antiretroviral therapy; HIV+: HIV positive; HIV-: HIV negative; IQR: interquartile range; MACS: Multicenter AIDS Cohort Study; n (%): number of individuals and percentage of sample; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; TTE: transthoracic echocardiogram; WIHS: Women’s Interagency HIV Study.
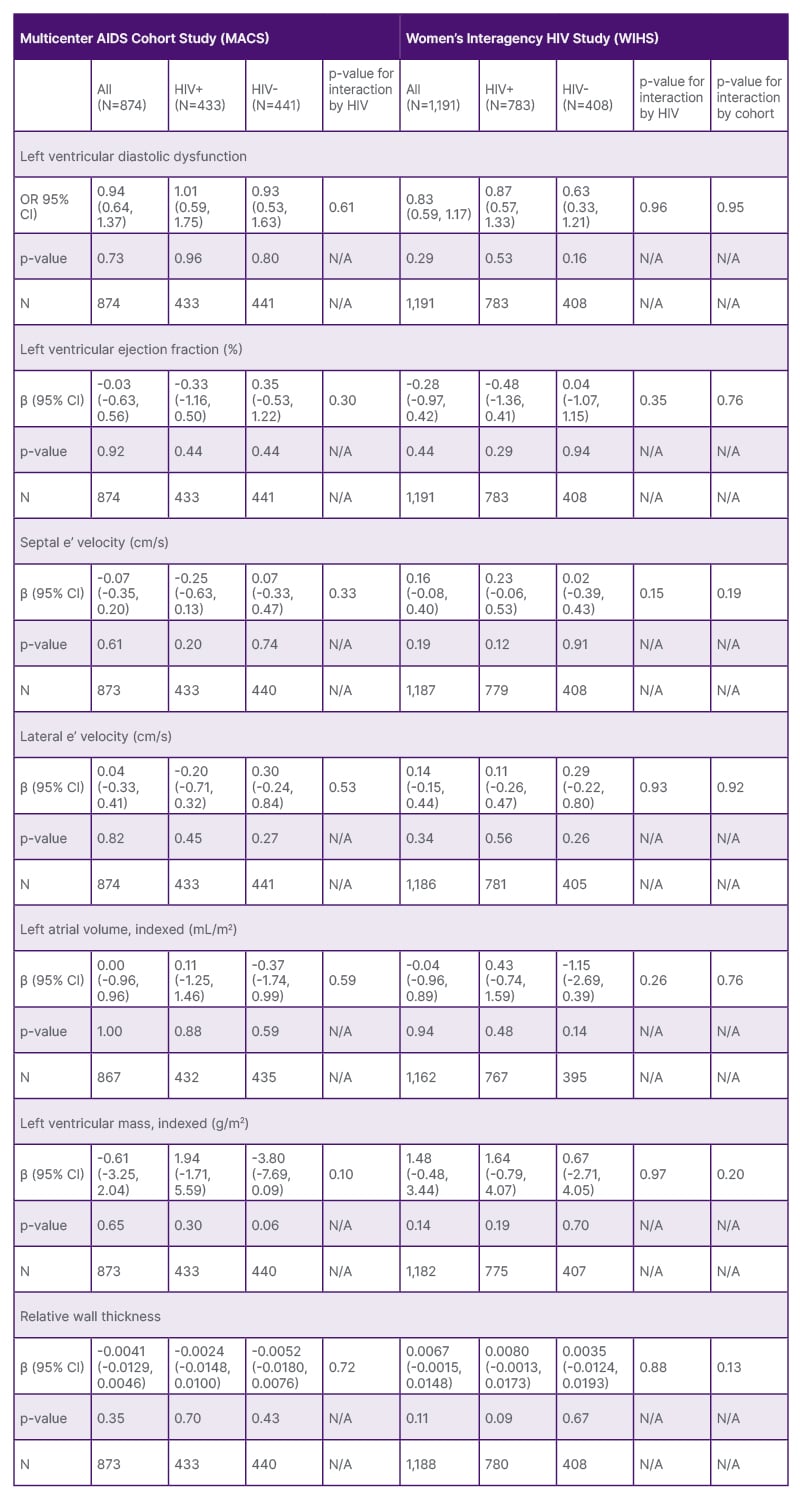
Table 3: Adjusted associations between depressive symptoms and indices of subclinical left ventricular dysfunction, by cohort and HIV status.
Final models were adjusted for race, site, smoking status, alcohol use since last visit, use of any recreational drug, HIV status, age at visit, body mass index at visit, hypertension status at visit, diabetes status at visit, and Hepatitis C status at visit. The analysis for the left ventricular diastolic dysfunction outcome excludes the 45 participants with left ventricular systolic dysfunction.
CHART: Characterizing Heart Function on Antiretroviral Therapy Study; OR: odds ratio.
DISCUSSION
This study examined the relationships between symptoms of depression and LVDD in two well-characterised, longstanding prospective cohorts of men and women living with and without HIV in the USA. Despite differences in sociodemographics and CVD risk factors among men and women in this study, no statistically significant differences were found by HIV serostatus status or by sex at birth in observed associations between depressive symptoms and the likelihood of LVDD, or with the individual components of the CHART criteria for LVDD.11 Findings suggest that while LVDD is prevalent among men and women with depressive symptoms, when controlling for sociodemographics, health, and other HIV related factors, depressive symptoms do not appear to be associated with LVDD.
In contrast with the current study, results from smaller previous studies identified that LVDD was correlated with major depressive symptoms23,24 in people living with and without HIV.25-27 This could be explained by the fact that the MACS and WIHS are long-lived cohorts in which most participants were well controlled, had been receiving antiretroviral therapy (ART), and who had documented depressive symptoms prior to the echocardiogram. As such, this sample included mostly persons who were on ART and/or had a high degree of virologic control but did not distinguish between depressive symptoms and MDD. In addition, previous reports did not compare heart function by HIV serostatus and by sex assigned at birth. This study comprises an important and novel contribution to the literature by evaluating associations between depressive symptoms and LVDD among participants by sex at birth and HIV serostatus. This analysis did not account for prior depressive symptoms or for the cumulative effect of depressive symptoms. Future research should consider examining these associations using measures of depressive symptoms that account for potential intensity and cumulative effects. Furthermore, in this study, there was a conflicting finding about ART status and LVDD between the cohorts. Specifically, the association between ART and LVDD was considerably negative among men (OR: 0.55) versus positive among women (OR: 2.45). As it appears that such a result has not been reported in the literature before, this finding warrants further research and examination to evaluate similar directionality in other groups. Finally, the use of newer ART now available (e.g., integrase strand transfer inhibitor-based regimens) should be evaluated in the future; this sample did not allow for further exploration of different types of ART, which may have a differential role on cardiac function.
Limitations of this study include that the data collected from this study was at a time when they were two different cohorts; therefore, the data was analysed for each of the cohorts. There were unique intrinsic differences in the cohorts including ethnicity and background differences. WIHS participants who were women were predominantly Black women from low socioeconomic backgrounds, many of whom were from the Southern USA (Alabama, Mississippi, Georgia, North Carolina, and Florida), where Medicaid expansion has not been adopted.28 Moreover, WIHS participants had fewer financial and medical resources than MACS participants, who were generally of higher income, higher education, not residing in the South, and predominantly White. Overall, MACS participants as a group were less subject to issues of systemic racism; these differences may have obscured results in the primary analyses and limited the generalisability of these findings. As such, these comparisons between cohorts (and therefore, comparisons by sex) may be biased by these broader cohort differences. Hence, there are many potential variables that can affect these findings. However, moving forward, the cohorts have unified, and the data is being harmonised, and each site will have both men and women enrolled in the MWCCS. Further research is needed to disentangle these relationships. Given the cross-sectional nature of this study design, inferences about causality cannot be made, and the potential for residual confounding and spurious associations remains despite adjustment for multiple covariates. A longitudinal study could offer more statistical power and enable the examination of the temporality of depressive symptoms and CVD outcomes, and vice versa.
This study has important strengths. A primary strength is the study population, which was drawn from two well-established ethnically and socially diverse cohorts; among whom over half were women. These longstanding cohorts utilised standardised data collection and intensively trained TTE testing, with centralised analyses, minimising potential information bias due to measurement error. Moreover, data from the ART era facilitates the understanding of depressive symptoms and subclinical heart structure and function in the context of virally suppressed HIV. Additionally, the inclusion of people living without HIV with otherwise similar risk phenotypes improved adjustment for potential confounders that may be associated with depression and alter cardiac structure and function.
CONCLUSION
In summary, this study examined the presence of depressive symptoms and TTE indices of LVDD among men and women both living with and without HIV. Depressive symptoms differed by gender and when compared by HIV status, men living with HIV had more depressive symptoms. However, the presence of depressive symptoms was not associated with LVDD. Longitudinal analysis could offer more statistical power and enable evaluation of the intensity and temporality of depressive symptoms and CVD outcomes, particularly in the context of HIV infection, in which current CVD clinical risk prediction tools remain suboptimal.







