Abstract
Diabetic macular oedema (DMO) is a common ocular problem among patients with diabetic retinopathy, which is sight-threatening and leads to blindness. The gold standard treatment for DMO had been focal/grid laser photocoagulation that achieved stabilisation of disease progression. However, newer pharmacological treatment options have gradually been favoured, as studies demonstrate their superior efficacy with regard to significant visual improvements. In particular, use of anti-vascular endothelial growth factor (anti-VEGF) has become very popular, with promising evidence emerging from numerous trials regarding efficacy and safety. Based on the 2014 American Society of Retina Specialists (ASRS) Preferences and Trends survey, the current preferred first-line therapy for DMO is in fact an anti-VEGF agent. Studies have shown that VEGF plays a critical role in both the angiogenesis and inflammation processes that occur during development of DMO. Hence, this allows anti-VEGF agents to specifically target and treat the underlying pathology, signifying its importance, and possibly accounting for its efficacy. We evaluate the available literature documenting the efficacy of anti-VEGF treatment in DMO. A key clinical finding was that anti-VEGF, as a drug class, achieved superior resolution of macular oedema and visual improvements that were consistently sustainable over 3 years, with some evidence pointing towards 5-year sustainability too. Hence, with intravitreal anti-VEGF treatments increasingly available, better long-term prognosis and, crucially, reduced likelihood of progression to blindness can be expected in patients with DMO.
INTRODUCTION
Diabetic macular oedema (DMO) is a common ocular problem among patients with diabetic retinopathy that is sight-threatening and leads to blindness. Being the leading cause of legal blindness in diabetics,1 effective management is crucial. Diagnosis is first made clinically, followed by quantification using optical coherence tomography and fundus fluorescein angiography for monitoring disease progression and treatment response.2 Currently, various treatments targeting different pathways in the pathogenesis of DMO exist, albeit with varying efficacy. In particular, anti-vascular endothelial growth factor (anti-VEGF) is fast becoming a popular treatment option over focal/grid laser photocoagulation (hereafter referred to as ‘laser’), the gold standard treatment for DMO over the past decade. Unlike laser that stabilises disease progression, anti-VEGF agents are reported to improve vision significantly.3 As VEGF is a critical molecule in the pathogenesis of DMO, this allows anti-VEGF agents to specifically target and treat the underlying pathology,4 signifying its importance. Hence, this review is focussed on discussing the effectiveness of anti-VEGF therapy in DMO, while associated safety and real-world cost concerns are discussed elsewhere.5
METHODS
A comprehensive literature search was conducted on Medline, PubMed®, and Cochrane® databases using the keywords: ‘anti-VEGF’, ‘bevacizumab’, ‘ranibizumab’, ‘aflibercept’, ‘trap-eye’, ‘pegaptanib’, ‘diabetic macular edema’. Only studies with abstracts and full-texts published in English between 2004 and 2016 were included. This time period was chosen to include seminal papers and landmark trials documenting the use of anti-VEGF in treating DMO. Selection of relevant articles was initially performed based on their titles and abstracts, followed by detailed review of their full-texts. In total, 28 clinical trials, 3 systematic reviews, 14 review articles, 5 retrospective studies, and 3 prospective studies were selected.
RATIONALE FOR ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR IN DIABETIC MACULAR OEDEMA
The associated vision loss in DMO occurs due to the breakdown of the blood-retinal barrier. Increase in vascular permeability results in plasma protein leakage and retinal swelling/thickening.4 Multiple studies have identified several growth factors and inflammatory mediators to be responsible for this breakdown, especially VEGF. Although the exact pathogenic mechanism is not completely understood, independent analysis by Fogli et al.4 supports the theory that VEGF plays a critical role in both the angiogenesis and inflammation processes that occur during the development of DMO, justifying the use of anti-VEGF in treatment. Currently, four different intravitreal anti-VEGF agents are used: namely, ranibizumab (RBZ), bevacizumab (BVZ), aflibercept, and pegaptanib. Their properties are summarised in Table 1. Their superior clinical efficacy reported in randomised controlled trials (RCT) (summarised in Table 2 and 3) and other studies, support a shift in treatment paradigm towards anti-VEGF.
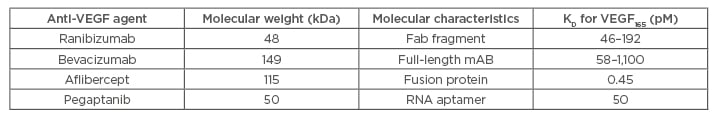
Table 1: Summary of molecular differences between anti-VEGF agents.
Fab: antigen binding fragment; KD: dissociation constant; mAB: monoclonal antibody; pM: picomolar; VEGF: vascular endothelial growth factor.
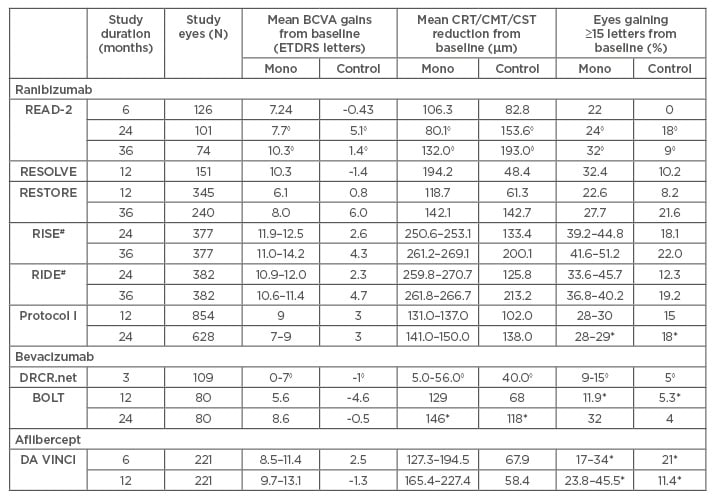
Table 2: Summary of main anatomical and functional outcomes in randomised controlled trials for ranibizumab, bevacizumab, and aflibercept.
◊Statistical analysis not available for results; *results not statistically significant (p>0.05); #results of open-label 2-year extension not included, because results were derived from pooled data across RISE/RIDE, making direct comparison with 24 and 36-week results reported in the Table meaningless.
BCVA: best-corrected visual acuity; CMT: central macular thickness; CRT: central retinal thickness; CST: central subfield thickness; ETDRS: Early Treatment Diabetic Retinopathy Study;
Mono: anti-VEGF monotherapy.
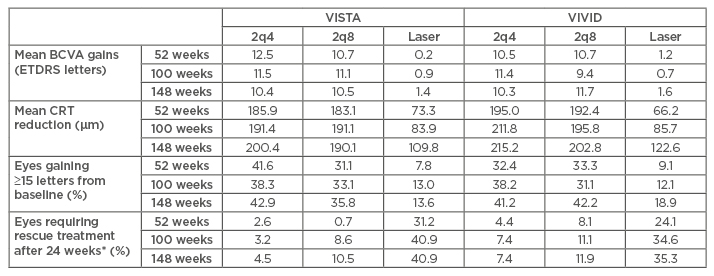
Table 3: Summary of 52 and 100-week results from VISTA/VIVID study.32-34
All results were clinically significant (p≤0.0001).
*Rescue treatment refers to the treatment modality that patient was not randomised to and have not received before (e.g., patients in the laser-treated group received rescue aflibercept of 5 doses 2q4 followed by 2q8 until end of study).
BCVA: best-corrected visual acuity; CRT: central retinal thickness; ETDRS: Early Treatment Diabetic Retinopathy Study; 2q4: 2 mg every 4 weeks after 5 initial monthly doses; 2q8: 2 mg every 8 weeks after 5 initial monthly doses.
Ranibizumab
RBZ (Lucentis™, Genentech, San Francisco, California, USA/Roche™, Basel, Switzerland) is a recombinant, humanised, monoclonal antibody fragment that binds all isoforms of VEGF-A. Currently, it is approved for treating age-related macular degeneration (AMD), retinal vein occlusion and diabetic retinopathy with or without DMO, in the USA and European Union (EU). Treatment regimens in DMO typically vary between practices.
Herein, we discussed five important RCT studying RBZ in DMO. Additionally, DRCR.net (Protocol T), comparing the efficacy of RBZ against two other anti-VEGF agents, will be separately discussed.
READ-2
A Phase II, 14-site, investigator-initiated clinical trial comparing 0.5 mg RBZ, laser, and 0.5 mg RBZ combined with laser over 6, 24, and 36 months. Six-month results demonstrated short-term visual and anatomical benefits of RBZ monotherapy, with mean best-corrected visual acuity (BCVA) improving significantly from baseline by +7.24 ETDRS (Early Treatment Diabetic Retinopathy Study) letters (versus -0.43 letters in laser), and 50% of this group experienced a reduction in foveal thickness (versus 33% in laser). Also, significantly more patients gained ≥15 letters with RBZ monotherapy (22%) than laser (0%).6 After 6 months (primary endpoint), most patients fulfilled the retreatment criteria and were retreated with RBZ monotherapy. Subsequent results revealed sustained benefits after 24 months in the monotherapy arm, while retreated patients experienced improved vision and reduction of residual oedema (Table 2).7 Despite improvements after 24 months, many patients continued to have persistent oedema, defined as central subfield thickness (CST) ≥250 µm, suggesting possible under-treatment. Consequently, treatment regimen was intensified from bimonthly to monthly, which saw significant improvements in both mean BCVA and CST (Table 2). Hence, suggesting that outcomes can be improved with more aggressive therapy.8
RESOLVE
A Phase II, multicentre, sham-controlled trial assessing the efficacy and safety of two doses of RBZ (0.3 mg and 0.5 mg) against placebo over 12 months. Dose effect was difficult to assess, due to study design allowing doubled doses of RBZ or rescue laser to be administered when needed (91.8% in placebo arm; 68.6% in RBZ arm). Hence, data reported was pooled from all RBZ-treated patients (regardless of dose) instead. Overall, RBZ-treated patients once again show improved outcomes compared to placebo (Table 2).9
RESTORE
A Phase III, multicentre trial comparing 0.5 mg RBZ, combination with laser, and laser over 12 and 36 months. Results after 12 months were similar to READ-2 and RESOLVE, demonstrating superiority of RBZ treatment over laser (Table 2).10 After 12 months, all remaining patients were treated with RBZ. Results after 36 months reported that visual and anatomical gains were sustained in the original RBZ arm, while previously laser-treated patients improved to similar levels as the original RBZ arm despite RBZ being delayed for a year (Table 2).11
RISE/RIDE
RISE/RIDE were two parallel, methodologically identical, Phase III, multicentre trials randomising patients into three groups (0.3 mg RBZ, 0.5 mg RBZ, and sham injections) to assess efficacy and safety of RBZ compared to sham over 3 years with an additional 2-year extension. Similar to RESTORE, all patients were changed onto RBZ therapy after 24 months. The 24 and 36-month results were very similar to RESTORE, with superior and sustainable ocular benefits reported in the RBZ arm after 3 years, and up to 54 months. However, among the originally laser-treated patients, delayed RBZ treatment never achieved a similar extent of visual improvements as the original RBZ arm after 36 and 54 months. This data therefore contradicts the extent of benefits seen in RESTORE (Table 2).12-14
DRCR.net (Protocol I)
The DRCR.net (Protocol I) study compared the efficacy of both steroid and RBZ treatment against laser over 5 years. Participants were divided into four groups (0.5 mg RBZ and prompt laser, 0.5 mg RBZ and deferred laser, 4 mg intravitreal triamcinolone and prompt laser, laser only). Of relevance, 1 and 2-year results demonstrated significant improvements in mean BCVA and CST reductions in both RBZ-treated groups compared to laser (Table 2) that was maintained through 5 years. Interestingly, both 3 and 5-year results reported reduced mean BCVA gains when laser was started at RBZ initiation (RBZ and prompt laser versus RBZ and deferred laser: +6.8 versus +9.7 letters [p=0.02, 3 years]; +7.2 versus +9.8 [p=0.09, 5 years]).15-18
Bevacizumab
BVZ (Avastin™, Genentech; Roche™) is a recombinant, humanised, full-length monoclonal antibody that binds all isoforms of VEGF-A. BVZ is currently US Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved for systemic treatment of several different cancers only, and its use for ocular diseases is off-label. Despite its off-label status, doctors commonly use it for wet AMD and DMO19 due to its low cost and efficacy demonstrated in various trials. Currently, no consensus on treatment regimen is available, with the most common being 1.25 mg monthly until oedema is stabilised before using an ‘as needed’ regimen.20
DRCR.net
The first Phase II, multicentre trial comparing various BVZ treatment regimens against laser identified several key findings.21 Firstly, eyes treated with intravitreal BVZ achieved significant BCVA improvement that persisted over 12 weeks, and greater CST reduction at 3 weeks compared to laser. Secondly, results identified no significant difference between the tested doses (1.25 mg, 2.5 mg). Thirdly, BVZ treatment achieved significantly greater BCVA improvements in DMO-treatment naïve eyes. Similar findings were also reported in a RCT conducted by Lam et al.22
BOLT
A Phase II, single-centre RCT, comparing 1.25 mg intravitreal BVZ and laser, provided further evidence supporting the benefits of BVZ. After 12 months, BVZ-treated patients gained a median of +8 letters (versus -0.5 letters in laser, p=0.0002) and mean central macular thickness (CMT) reduction of -129 µm (versus -68 µm in laser, p=0.02). Similar functional and anatomical improvements were also reported at 24 months (Table 2).23,24
PACORES
A recently published 5-year result from a multicentre retrospective study conducted in Latin America and Spain seemed to disagree about the sustainability of visual gains after 5 years.20 The study included eyes treated with at least one injection of 1.25/2.5 mg of intravitreal BVZ with an ‘as needed’ regimen. Results showed that mean BCVA ultimately returned to baseline after 60 months, despite significant improvements at 36 months. Subgroup analysis showed that after 60 months about 75% of BVZ-treated patients had at least stable BCVA, even though only 29% achieved visual gains. Notably, significant anatomical improvements were observed 6 months after treatment and remained relatively constant throughout the 60 months.
Other studies
A review of intravitreal BVZ in DMO looked at several smaller studies with different study parameters, and similarly concluded its general efficacy.19 Two noteworthy points not seen in RCT, due to their strict exclusion criteria, are that BVZ seemed to achieve visual and anatomical improvements even in cases that were unresponsive to other DMO treatments,25,26 but not in cases with macular ischaemia.27 However, these respective studies are inconclusive due to short follow-up periods.
Aflibercept
Aflibercept (Eylea™, Regeneron, Tarrytown, New York, USA, formerly VEGF trap-eye) is a high affinity, recombinant fusion protein, with VEGF-binding domains of human VEGF receptors 1 and 2 fused to fragment crystallisable domain of human immunoglobulin-G1, which binds all circulating VEGF isoforms and placental growth factor. It is currently approved in the USA, EU, Japan, and Australia for several different vascular ocular diseases, including DMO.28 The recommended dose is 2 mg every 8 weeks after 5 initial monthly loading injections.28 Three landmark trials were conducted with convincing results supporting intravitreal aflibercept in DMO.
DA VINCI
A multicentre, active-controlled RCT, compared four different aflibercept regimens to laser, and each regimen achieved statistically significant improvements. The 24-week results showed aflibercept subgroups achieving mean BCVA gains between +8.5 and +11.4 letters (versus +2.5 letters in laser, p≤0.0085 for each subgroup), and mean CRT reduction between -127.3 µm and -194.5 µm (versus -67.9 µm with laser, p=0.0066 for each subgroup).29,30 The superiority of aflibercept over laser remained statistically significant even after 1 year (Table 2).31
VISTA/VIVID
Two similarly designed Phase III, active-controlled RCT, comparing two aflibercept regimens (2 mg every 4 weeks and 2 mg every 8 weeks after 5 initial monthly doses) to laser were carried out. The 52, 100, and 148-week results are summarised in Table 3. Results after 1 year were very similar to those reported in the DA VINCI trial for three outcome measures. Additionally, the reported 100 and 148-week results provided strong significant evidence of the sustainability of visual benefits with aflibercept over laser even in the longer term. Additionally, the study design allowed all patients who met retreatment criteria to be given rescue treatment after Week 24. Aflibercept-treated eyes consistently required significantly less rescue treatment compared to laser-treated eyes from 24 to 148 weeks. Overall, no significant differences in efficacy were found between the two aflibercept regimens.32-34
Pegaptanib
Pegaptanib sodium (Macugen™, Eyetech™, New York City, New York, USA) is a ribonucleic acid aptamer that specifically blocks the ocular angiogenic activity of the VEGF165 isoform. Although it is approved only for the treatment of wet AMD in USA and Europe,35 results from Phase II and III trials are highly supportive of its application in treating DMO. Both trials recommend a dosage regimen of 0.3 mg every 6 weeks followed by an ‘as needed’ regimen.
A Phase II RCT reported both functional and anatomical improvements with 0.3 mg compared to sham. Median BCVA at Week 36 was significantly better, with 0.3 mg (20/50) compared to sham (20/63) (p=0.04), and significantly more patients gained VA of ≥10 letters with 0.3 mg (34%) compared to sham (10%) (p=0.003). Treatment reduced mean central retinal thickness (CRT) by -68 µm, while sham resulted in +4 µm thickening (p=0.02).36
Findings drawn from 2-year data collated from another Phase II/III trial confirmed the short term clinical benefits and suggested continued long-term visual improvements. After Week 54, 36.8% of patients treated with pegaptanib gained BCVA of ≥10 letters compared to 19.7% with sham (p=0.0047). At Week 102, the same BCVA improvement was higher in the treatment group (38.3%) compared to sham (30.0%), albeit not statistically significant (p=0.1729). Additionally, the trial showed statistically superior improvements in mean VA from baseline after treatment compared with sham (p<0.05) at both Weeks 54 and 102. However, CRT improvements between both groups at Weeks 54 and 102 were ‘numerically different but not statistically significant’ suggesting that benefits of pegaptanib might be more functional rather than anatomical.37
A separate meta-analysis combined data from both of these trials and demonstrated a statistically significant greater percentage of treated patients gained VA of ≥15 letters over sham.38 Also, two smaller studies (one prospective and one retrospective) both reported significant improvements in mean BCVA and mean CRT with pegaptanib too,39,40 indicating effectiveness across a wider population.
COMPARISON BETWEEN ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS
DRCR.net (Protocol T) is the first and only RCT directly comparing more than two different anti-VEGF agents, enabling relative efficacy to be studied. This multicentre RCT randomised patients into three groups (0.3 mg RBZ, 1.25 mg BVZ and 2 mg aflibercept) with BCVA gains as the primary outcome measure. Mean BCVA gains from baseline were +11.2, +9.7, and +13.3 letters for RBZ, BVZ, and aflibercept, respectively, after 1 year, and +12.3, +10.0, +12.8, respectively, after 2 years. Overall, all three anti-VEGF agents were efficacious in DMO. Subgroup analysis identified that in eyes with better baseline BCVA (between 20/32 and 20/40), 1-year and 2-year BCVA gains were similar across the three agents. However, in eyes with poorer baseline VA (20/50 or worse), aflibercept performed significantly better than BVZ and RBZ after 1 year, but only superiority over BVZ was maintained after 2 years. Notably, no significant difference in BCVA improvements was found between RBZ and BVZ over the 2 years.28,41,42
DISCUSSION
The use of anti-VEGF agents in DMO has popularised over the last few years. Before that, the gold standard treatment was laser that reduces the 3-year risk of moderate vision loss by 50%, compared to observation in the landmark ETDRS study.43 However, as the disease may progress to blindness, settling for a treatment that slows DMO progression is suboptimal. Ideally, treatment should improve visual outcomes early on and persist in the long run to be considered effective, due to disease chronicity requiring prolonged repeated treatments.
Unfortunately, laser only improved vision by three lines in 11% and 16% of patients after 1 and 3 years, respectively.43 Comparatively, results from the aforementioned landmark studies clearly highlight the superior therapeutic benefits of anti-VEGF agents in achieving both visual improvements and resolution of DMO. Overall, as a drug class, early clinical benefits are observed as early as 1 month.20 Furthermore, current evidence strongly supports the sustainability of clinical benefits for at least 3 years, with several RCT, like RISE/RIDE and Protocol I, even reporting persistent improvements after 5 years. However, two retrospective studies presenting 5-year data, the PACORES study and Wecker et al.,44 both reported mean BCVA returning to baseline with no significant improvements after 5 years.20,44 These conflicting conclusions in 5-year sustainability might possibly be accounted for by differences in study population characteristics. Is the heterogeneous sample in these retrospective studies a better reflection of real-world patient characteristics? Or is there an inherent selection bias affecting how we should interpret long-term results from these retrospective studies?44 Hence, we believe that 5-year benefits are likely to be sustainable in most patients, but not necessarily in patients with resistant/recurrent DMO. Nonetheless, in such patients, anti-VEGF treatment is likely to be non-inferior to laser after 5 years, as further deterioration towards blindness is avoided; hence, making anti-VEGF therapy superior and more popular than laser.
Additionally, encouraging retreatment results show that patients can still benefit from anti-VEGF therapy, even if initiation was delayed. However, whether delayed anti-VEGF therapy can achieve levels of improvement seen in early treatment is still debatable. Chung et al.27 suggested avoiding BVZ treatment in patients with macular ischaemia as it led to worse visual outcomes. This is particularly interesting as it would constitute a contraindication for anti-VEGF treatment. A possible explanation could be that the downregulation of VEGF below physiological levels induces excessive vasoconstriction, that further disrupts the already compromised chorioretinal circulation in diabetics, causing macular ischaemia and poorer visual outcomes.27,45 Though such data is limited, another review suggests the rare possibility of anti-VEGF compromising retinal circulation,45 thereby causing macular ischaemia. Hence, extra precautions, such as frequent follow-ups or alternative treatments, should be considered in such patients until conclusive evidence is found.
In terms of the drug class effect among anti-VEGF agents, two articles reviewed both reported visual and anatomical improvements after converting from RBZ/BVZ to aflibercept treatment.46,47 Hence, it is worth switching between anti-VEGF agents if response is suboptimal. This makes close monitoring early on after initiation especially important. Nonetheless, more studies are required to fully understand whether similar improvements are achieved when converting to other anti-VEGF agents besides aflibercept.
CONCLUSION
In conclusion, intravitreal anti-VEGF agents have definitely revolutionised the management of DMO. The reported superior visual improvements and resolution of macular oedema create a strong case for it to be the new standard of care. Nonetheless, caution should be taken when treating patients with macular ischaemia or persistent DMO. Hopefully, with long-term clinical benefits of anti-VEGF therapy generally sustainable, prevalence of DMO can be effectively lowered to reduce blindness.








