Abstract
Introduction: Biomarkers of prognostic and predictive relevance are needed for the practical management of COVID-19.
Objective: The authors aimed to assess a battery of inflammatory cytokines in patients with SARS-CoV-2 to determine the cytokines of prognostic and predictive relevance in COVID-19.
Methods: In a cohort of 100 patients with SARS-CoV-2 (RT-PCR confirmed), hospitalised in Shri Maharaja Hari Singh Hospital associated to Government Medical College Srinagar, India, the level of a battery of cytokines, IL-6, IL-8, IL-10, IL-1α, vascular endothelial growth factor (VEGF), TNF-α, and ferritin were estimated by ELISA on a multimode microplate reader.
Results: The deranged levels of these cytokines were mostly found in patients >60 years of age, with cough and pneumonia as the most common symptoms. A significant association was found between IL-6 and IL-8, disease severity (p=0.002; p=0.007), and poor disease outcome (p=0.040; p=0.009), respectively. A significant association was also found between decreased levels of VEGF and poor disease outcome (p=0.020). Further receiver operating characteristic analysis, univariant and multivariant (after adjusting for age, gender, and other inflammatory markers), revealed increased IL-10 (area under the curve [AUC]: 0.72) and IL-6 (AUC: 0.70) as independent markers of both disease severity (p=0.02; p=0.01) and disease outcome (P=0.03; p=0.02), respectively, and decreased VEGF (AUC: 0.69) as an independent marker of disease outcome (p=0.03). A significant association between cough and IL-8 (p=0.01) and IL-10 levels (p=0.03), and of diabetes and raised ferritin levels (p=0.01), with very high ferritin levels (>1500 ng/mL), was found in those who are likely to develop hyperinflammatory phenotype.
Conclusion: The authors conclude that ‘IL-6, IL10, VEGF, and IL-8’ are the signature inflammatory cytokine panel/profile in COVID-19, particularly in patients from Kashmir. Increased IL-10 and IL-6 levels proved to be equally significant independent prognosticators of COVID-19 severity and outcome, and decreased VEGF levels were independent predictors of poor disease outcome in patients with SARS-CoV-2. Testing of the signature inflammatory cytokine panel is, therefore, recommended for optimal clinical decision-making in patients with COVID-19 from Kashmir, India.
Key Points
1. Cytokine storm is the key pathophysiology underlying COVID-19.2. In a cohort of 100 hospitalised patients with COVID-19 from Kashmir, India, cytokine profiling was carried out and correlated (by univariate and multivariate analysis) with COVID-19 severity and outcome.
3. The authors found ‘IL-6, IL10, vascular endothelial growth factor, and IL-8’ as particularly significant inflammatory cytokines of prognostic and predictive relevance. Testing of this cytokine panel is, therefore, recommended for clinical decision-making at least in patients with COVID-19 from Kashmir, India.
INTRODUCTION
COVID-19 is a novel β-coronavirus caused by infection with SARS-CoV-2. It is the third zoonotic disease caused by coronavirus to affect humans, following severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).1,2 Globally, 775,615,736 confirmed SARS-CoV-2 cases and 7,051,323 deaths have been reported by the World Health Organization (WHO; as of June 2024). Symptoms of COVID-19 typically become noticeable after a 5–6-day incubation period and commonly consist of fever, cough, and fatigue. Other potential symptoms may include headache, haemoptysis, diarrhoea, dyspnoea, and leukopenia, among other signs.3,4 Elderly patients and those with pre-existing conditions such as high blood pressure, chronic obstructive pulmonary disease, diabetes, or cardiovascular disease are more likely to develop acute respiratory distress syndrome, septic shock, metabolic acidosis, coagulation dysfunction, arrhythmia, kidney damage, heart failure, liver dysfunction, and/or secondary infection, which can often lead to death.5,6
Disruption of immune homeostasis is the primary cause of mortality, morbidity, and post-COVID syndromes associated with COVID-19. Recent studies have shown that infection spike glycoprotein of SARS-CoV-2 is actually recognised or sensed by toll-like receptor 4 (TLR4), which is a type of pattern recognition receptor found on innate immune cells like circulating monocytes, macrophages, and dendritic cells. This binding activates downstream signalling pathways via MyD88, NF-κB, or interferon receptor factors that determine the expression of proinflammatory cytokines. The in situ TLR4 activation in alveolar macrophages leads to intense local inflammation, resulting in the accumulation of inflammatory factors that result in the overactivation of the innate adaptive immune response, leading to over-immunopathology known as cytokine storm. Cytokine storm ultimately causes respiratory gas exchange, breathing problems, and sometimes even death.7-9 Immune-active molecules such as IL have been suggested to contribute to the development of cytokine storm. Among the inflammatory cytokines, IL-8 is a powerful pro-inflammatory cytokine crucial in recruiting and activating neutrophils during inflammation and plays a role in the pathophysiology of COVID-19.10 Besides, IL-6 and IL-10 are other highly active inflammatory cytokines that have been associated with rapid disease progression and higher complication risk in COVID-19.11,12 Despite the information available about some inflammatory predictive and prognostic cytokines in COVID-19, it has been observed that people from geographically diverse regions and of different ethnicities and genetics behave differently against the SARS-CoV-2 virus. While some can resolve it quickly, others succumb to the disease. Furthermore, there is a possibility that SARS-CoV-2 could modify host innate immune responses to avoid immune identification and weaken human defences. To find these differences, which may be unique to the population, it is essential to assess the inflammatory cytokine response in patients with SARS-CoV-2 from this part of the world. Defining the standard ranges for different cytokines and their association with clinical findings may result in the identification of some valuable biomarkers as indicators of COVID-19. The present study, therefore, aimed to evaluate the prognostic and predictive value of candidate inflammatory (pro and anti) markers in patients with SARS-CoV-2 from Kashmir, India.
MATERIALS AND METHODS
The study was conducted in the Department of Biochemistry, Government Medical College, Srinagar, India, and its associated hospitals between October 2020–November 2021. A total of 100 patients (RT-PCR confirmed SARS-CoV-2 positive) and 20 control subjects (RT-PCR confirmed SARS-CoV-2 negative) were included in this cohort study. The patients were diagnosed as per standard WHO/Centers for Disease Control and Prevention (CDC) criteria 2020 for COVID-19 disease. The study was initiated after obtaining approval from the Ethical Committee of Government Medical College, Srinagar (IEC/GMC-Sgr/27, 19th December). Written informed consent and response questionnaires from patients and healthy controls were documented and recorded as per hospital protocol. The study was hospital-based and included patients who were admitted to the hospital for COVID-19 complications. The samples were collected during the first week of hospitalisation, and the patients were then followed twice on the 14th day and 28th day of their admission. The clinical staging was as per the National Institute of Health’s (NIH) guidelines.13 Individuals who showed evidence of lower respiratory disease during clinical assessment or imaging, and had an oxygen saturation measured by pulse oximetry (SpO2) ≥94% on room air at sea level were classified as moderately ill (Stage 2). Individuals who had SpO2 <94% on room air, at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, a respiratory rate >30 breaths/min, or lung infiltrates >50% were classified severely ill (Stage 3). Individuals who had respiratory failure, septic shock, and/or multiple organ dysfunction, and later failed to survive, were classified as critically ill (Stage 3). So, as far as the treatment is concerned, in this cohort, 52% of patients received piperacillin and tazobactam, 82% received dexamethasone, 47% received azithromycin, 83% received enoxaparin, and 27% received remdesivir.
The serum samples collected were stored at -70 °C until further investigations. The levels of IL-6, IL-8, IL-1α, IL-10, VEGF, TNF-α, and ferritin were estimated by ELISA on a multimode microplate reader using kits of Diaclone (DIACLONE SAS, part of Medix Biochemica, Besançon Cedex, France) or on an automated immunoanalyser (Siemens, Munich, Germant). Twenty RT-PCR-confirmed SARS-CoV-2 negative subjects were taken as the control group to determine the reference range of the cytokines which were not available.
Statistical Analysis
The data were analysed using STATA software 17 (standard edition; StataCorp LLC, Lakeway Drive, College Station, USA). Correlation analysis of cytokines with socio-demographic, clinical features, comorbidities, clinical stages, and outcomes was determined by Chi-square test and Fisher exact test. Receiver operating characteristic analysis (univariate and multivariate) of various cytokines was done to determine the prognostic and predictive values of the cytokines after adjusting for various confounders.
RESULTS
Association of Inflammatory Cytokine Levels with Clinical Features and Comorbidities in Patients with SARS-CoV-2
In this cohort study, elevated levels of IL-10, IL-6, IL-8, IL-1α, TNF-α, ferritin, and decreased levels of VEGF were found typically in patients aged >60 years. Further association of inflammatory cytokines (IL-10, IL-8, IL-6, IL1-α, VEGF, TNF-α, and ferritin) with clinical features (Supplementary Table 1A and 1B) and comorbidities (Supplementary Table 2A and 2B), revealed significant association of cough with IL-8 levels (p=0.01) and IL-10 levels (p=0.03), and of diabetes with raised ferritin levels (p=0.01).
Association of Inflammatory Cytokines with Disease Severity/Staging and Disease Outcome in Patients with SARS-CoV-2
The association of inflammatory cytokines with disease severity and outcome in patients with SARS-CoV-2 revealed significantly elevated levels of IL-8 (p=0.007; p=0.009) and IL-6 (p=0.002; p=0.040) in patients of increased disease severity and poor outcome, respectively (Figures 2B and 2D; Table 1). Further, a significant association between decreasing VEGF levels and poor disease outcomes was found (p=0.02; Table 1). An association, though not significant, between decreasing VEGF levels and increased disease severity was found in patients (Figure 2F).
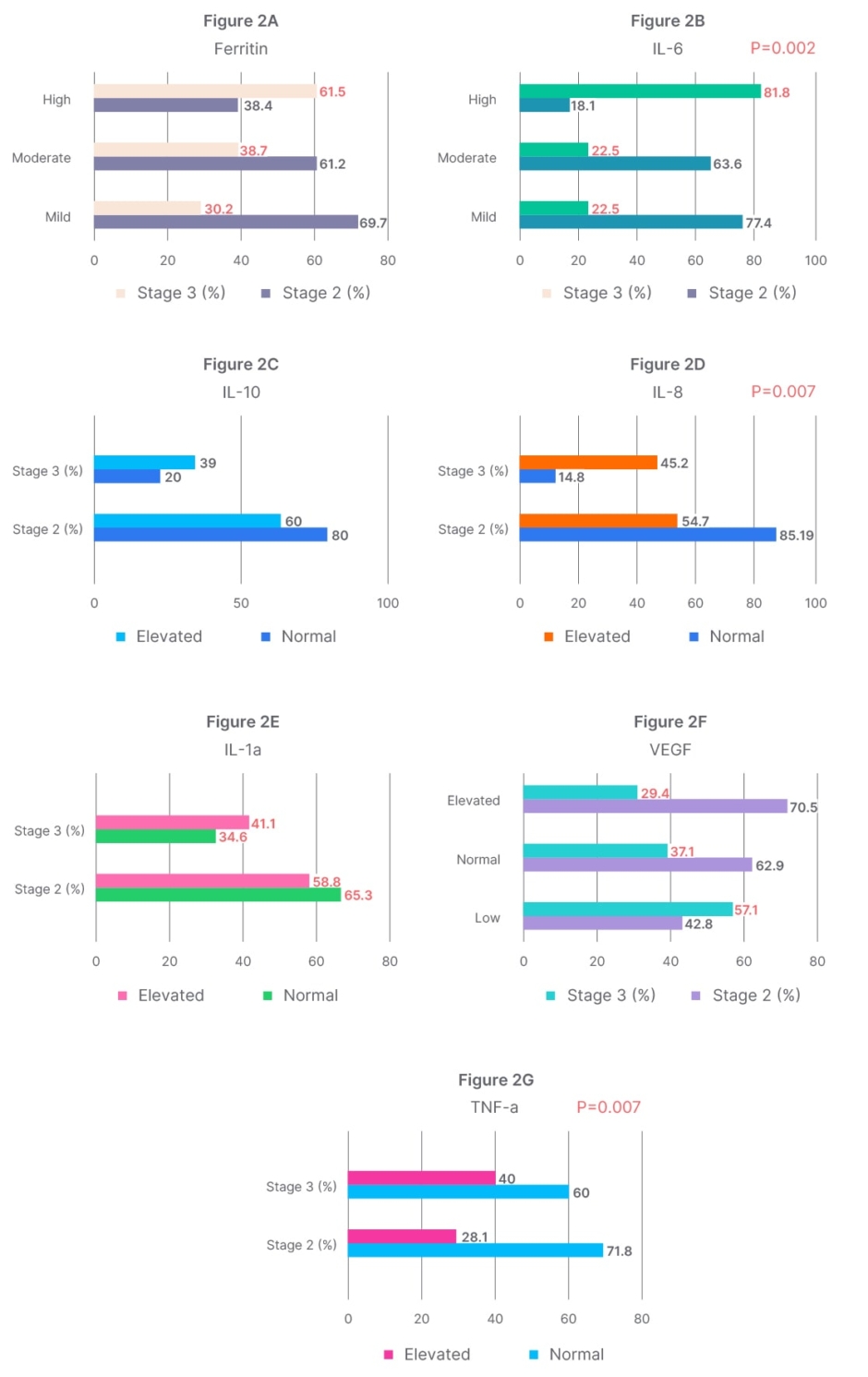
Figure 2A–G: Correlation analysis of cytokine, IL-10, IL-6, IL-8, IL-1α, TNF-α, vascular endothelial growth factor, ferritin levels and disease severity/staging in patients with SARS-CoV-2.
Figures (2A–G) represent correlation analysis of cytokines, IL-10, IL-6, IL-8, IL-1α, TNF-α, VEGF, ferritin, and disease severity/staging in SARS-CoV-2 patients.
The clinical staging was as per the National Institute of Health (NIH) guidelines.13
Stage 2 (moderately ill): individuals who show evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation measured by pulse oximetry (SpO2) ≥94% on room air at sea level.
Stage 3 (severely ill): individuals who have SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mmHg, a respiratory rate >30 breaths/min, or lung infiltrates >50%.
Stage 3 (critically ill): Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction, which later failed to survive.
VEGF: vascular endothelial growth factor.
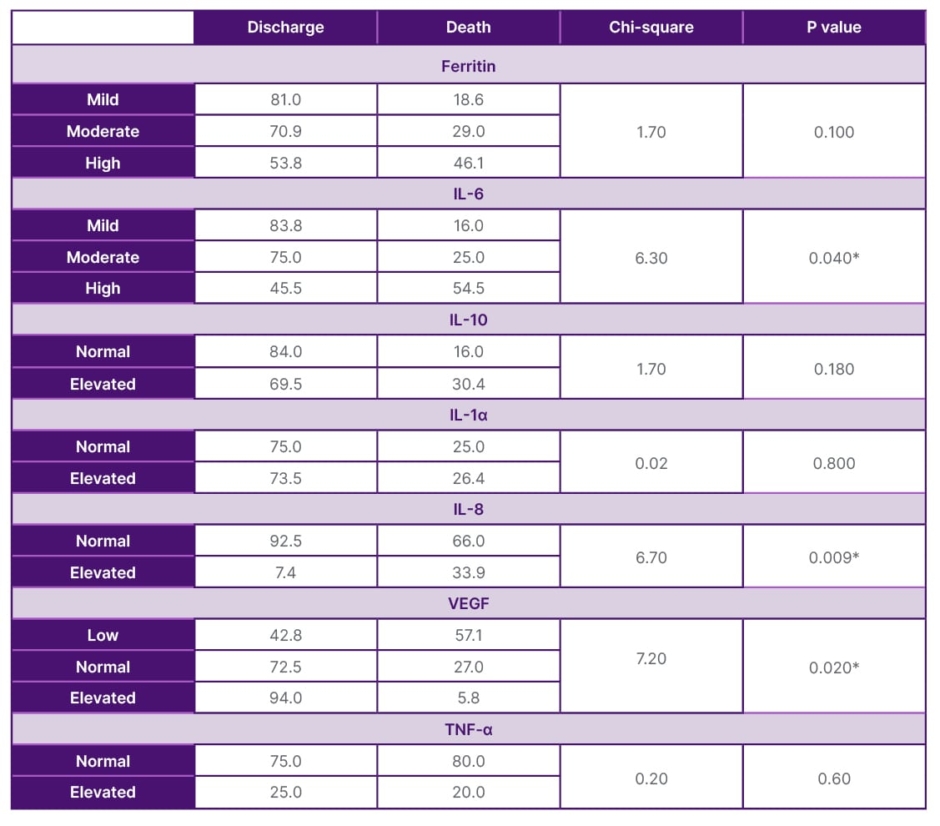
Table 1: Association of inflammatory cytokine IL-10, IL-6, IL-8, IL-1α, TNF-α, VEGF, and ferritin levels with disease outcome in patients with SARS-CoV-2 (N=100).
*Values considered significant when p≤0.05.
VEGF: Vascular endothelial growth factor.
Receiver Operating Characteristic Analysis of Inflammatory Cytokines with Disease Outcome in Patients with SARS-CoV-2
In determining the predictive value of cytokines (IL6, IL-1α, IL-10, IL8, VEGF, TNF-α, and ferritin), receiver operating characteristic analysis of these inflammatory cytokines with disease outcome (followed twice on the 14th and 28th day) revealed raised IL-10 (area under the curve [AUC]: 0.72, at the sensitivity of 68% and specificity of 72%) and IL-6 (AUC: 0.70, at sensitivity of 70% and specificity of 62%) as equally significant predictive markers of poor disease outcome, followed by IL-8 (AUC: 0.67), TNF-α (AUC: 0.66), and ferritin (AUC: 0.66), and decreased VEGF level as predictors of poor disease outcome (AUC: 0.69, at sensitivity of 53% and specificity of 73%) (Figure 1).
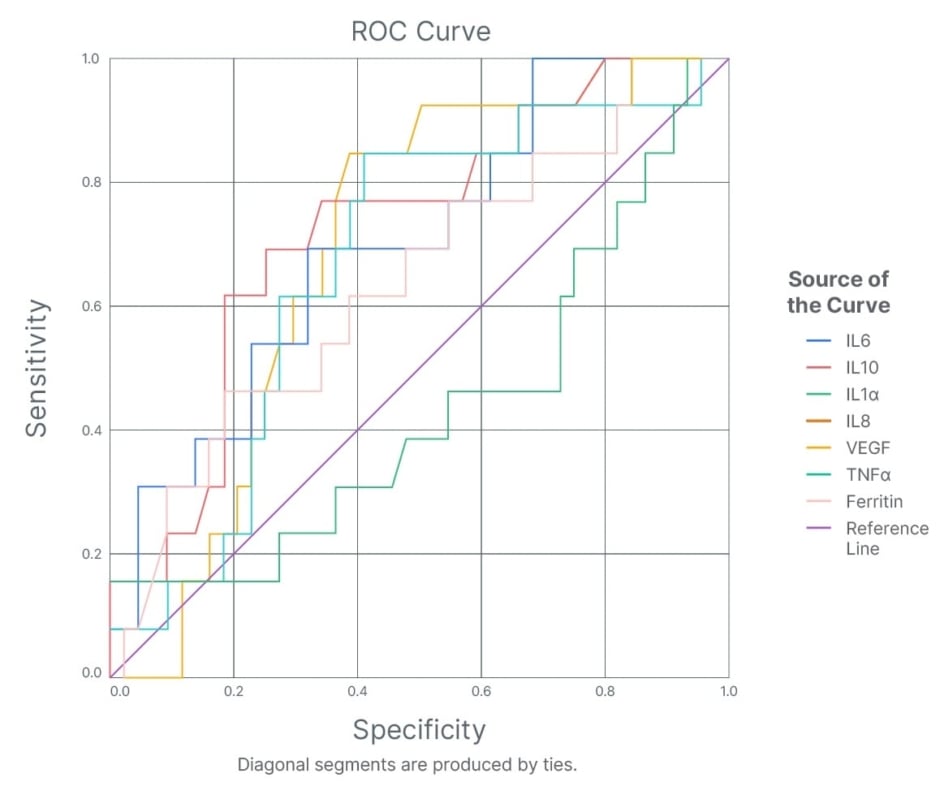
Figure 1: Receiver operating characteristic analysis of inflammatory cytokines, IL-10, IL-6, IL-8, IL-1α, TNF-α, vascular endothelial growth factor, and ferritin with disease outcome in patients with SARS-CoV-2.
ROC: receiver operating characteristic; VEGF: vascular endothelial growth factor.
Multivariate Analysis of Inflammatory Cytokines IL-10, IL-6, IL-8, IL-1α, TNF-α, VEGF, Ferritin, with Disease Severity and Outcome
Using multivariate analysis models, after adjustments of age, gender, and other inflammatory markers, increased IL-6 and IL-10 levels were found to be independent predictors of disease severity and poor disease outcome (Table 2), and decreased VEGF levels to be an independent predictor of poor disease outcome in COVID-19 (Table 3).
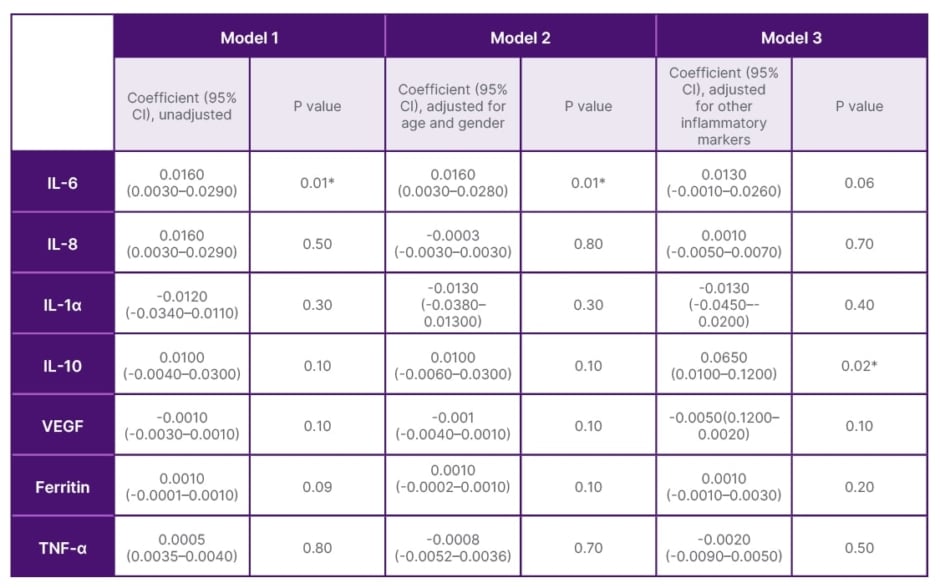
Table 2: Multivariate analysis of inflammatory cytokines, IL-10, IL-6, IL-8, IL-1α, TNFα, vascular endothelial growth factor, ferritin with disease severity/stages (II/III).
*Values considered significant when p≤0.05.
VEGF: vascular endothelial growth factor.
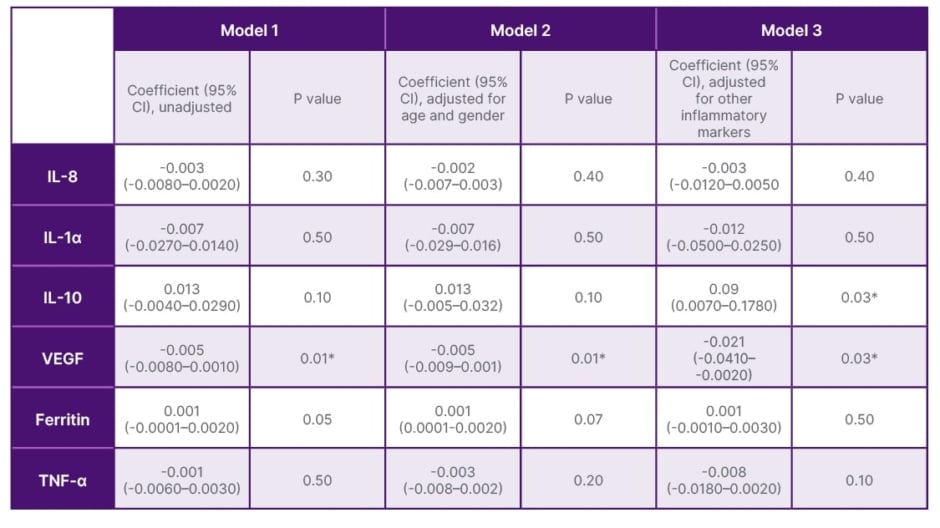
Table 3: Multivariate analysis of inflammatory cytokines, IL-10, IL-6, IL-8, IL-1α, TNFα, vascular endothelial growth factor, ferritin markers with disease outcome (death/discharge).
*Values considered significant when p≤0.05.
VEGF: vascular endothelial growth factor.
DISCUSSION
Given the significance of inflammatory cytokines in predicting disease severity and outcome in patients with SARS-CoV-2, the present study evaluated a battery of inflammatory cytokines in patients with SARS-CoV-2 from Kashmir, India and found a rise in IL-8, IL-10, and IL-6, and a decrease in VEGF as particularly significant signature inflammatory cytokine panel, as prognosticators of disease severity, and/or predictors of poor disease outcome.
Cytokine storm is a key feature of the SARS-CoV-2 infection. Upon infection binding of the SARS-CoV-2 spike protein to angiotensin-converting enzyme on Type II pulmonary alveolar epithelial cell, the damage-associated molecular patterns present on the virus get sensed by pattern recognition receptors like TLR4, which are present on innate and adaptive immune cells in alveolar cells like the macrophages, monocytes, T and B cells, and activates various signalling cascades, resulting in the production of pro-inflammatory cytokines. The in situ TLR4 activation by spike proteins of SARS and invasion of the inflammatory immune cells in the alveoli cause lung inflammation and lung damage.7-9,14-16 Studies have shown that inflammatory cytokines play a pivotal role in the pathogenesis of severe COVID-19. Regarding IL-6, the findings from the present study are inconsistent with the meta-analysis (nine studies) that reported an over three-fold increase (≥80 pg/mL) in IL-6 levels as a mortality risk factor in SARS-CoV-2 infection.17 The efficacy of the dexamethasone and anti- IL-6 treatment in COVID-19 could be explained by the underlying mechanism of reduction of IL-6 levels in patients with moderate and severe SARS-CoV-2 as proved by Ledford.18 Consistent with worldwide studies, ferritin here has been found to be a prognosticator of COVID-19 severity, and very high ferritin (>1500 ng/mL) levels as an indicator of hyperinflammatory phenotype.19 The possible mechanism is that hyperferritinemia causes inflammatory states in SARS-CoV-2 infection, particularly in the lungs, as demonstrated by the presence of a high number of macrophages in the lung parenchyma of patients with SARS-CoV-2. Furthermore, high ferritin also potentiates the production of proinflammatory cytokine IL-6, as a feedback mechanism exists between ferritin and IL-6.20
Similar to IL-6, here IL-10 proved to be an equally significant independent predictor of disease severity and poor disease outcome after nullifying the risk contribution of variables like age, gender, and other inflammatory markers for COVID-19. The finding is consistent with various other studies, including that of Lu et al.21 and Aber et al.22 IL-10 is a pleiotropic cytokine produced by various immune cells with strong anti-inflammatory and immunosuppressive effects. It typically suppresses pro-inflammatory signals by activating the JAK1-TYK2-STAT3 pathway, leading to STAT3-mediated transcription of genes that limit the inflammatory response, as in COVID-19.23,24 During the acute phase of viral infection, IL-10 suppresses the activity of T cells, macrophages, and natural killer cells, by preventing successful viral elimination and minimising collateral tissue damage. However, during the acute phase of SARS-CoV-2 infection, and in rare cases, such as in patients with comorbidities (e.g., diabetes, cancer, those on immunosuppressants, or elderly patients), very high levels of IL-10 have been observed, impairing its anti-inflammatory function and instead potentiates pro-inflammatory action of various cytokines. High levels of IL-10 are also known to augment the proinflammatory reactions to bacterial lipopolysaccharides in human endotoxemia.25 This points to the possibility that the combination of elevated IL-10 and bacterial products (which are abundant in severe COVID-19 cases) could empower the inflammatory machinery in COVID-19.26 Consistent with the present study, high IL-10 levels found in patients with comorbidities may provide a potential link between diabetes and negative outcomes in COVID-19.27 The present study, therefore, reaffirmed the importance of considering age and comorbidities while relating the cytokines with disease severity in COVID-19.
IL-8 is normally secreted by multiple cells, including neutrophils exposed to stimuli, and is considered the primary molecule of acute inflammation. Similar to the present study, several studies have shown neutrophilia and increased neutrophil–lymphocyte ratio as indicators of COVID-19 severity, so IL-8 is raised.28 Since IL-8 is expressed by neutrophils, therefore, consistent with the present study, IL-8 overexpression, by potentiating neutrophil chemotaxis and promoting angiogenesis, proves to be a poor prognostic biomarker for patients with SARS-CoV-2.
VEGF, apart from promoting angiogenesis, also effectively increases vascular permeability through the VEGFR2 receptor-mediated alterations of vascular fenestration and inter-endothelial junction.29 During a COVID-19 infection, patients frequently experience dyspnoea, which induces the activation of hypoxia-inducible factor-1-alpha among which VEGF is one of the key HIF-targeted genes.30,31 Consistent with other inflammatory markers, an early rise in VEGF levels has been found; however, a decreasing trend in VEGF with severity has been observed here in COVID-19. The present study was limited by being single-centric and included only a small number of samples. The results of the study, therefore, should be substantiated by similar multicentric studies from various parts of the country and even from other countries conducted on a large sample size. Also, the reference range of some of the cytokines was not known, so samples from healthy controls were taken in order to determine the reference range.
Taken together, the authors’ results reinforce the importance of closely monitoring the levels of cytokine panel IL-6, IL-10, VEGF, and IL-8, which might provide valuable information about the progression and changes during the treatment process of COVID-19 at the earliest.
CONCLUSION
The authors conclude that ‘IL-6, IL-10, VEGF, and IL-8’ are the signature inflammatory cytokine panel in COVID-19. Increased IL-10 and IL-6 levels proved to be equally significant independent prognosticators of COVID-19 severity and predictors of poor outcome and decreased VEGF levels as independent predictors of poor disease outcome in patients with V-2. Considering the success of anti-IL-6 targeted therapy, profiling of the present signature inflammatory cytokine panel is, therefore, suggested as part of the inclusion criteria for optimal clinical decision-making in patients with COVID-19.







