Meeting Summary
Individuals with diabetes often face challenges in managing diabetes-related tasks such as glucose monitoring, insulin treatment, and maintaining glycaemic control, leading to a psychosocial burden. Integrating new devices and technologies into diabetes care is crucial to improve quality of life.
During the American Diabetes Association (ADA) Scientific Sessions in Orlando, Florida, USA, on 21st–24th June 2024, Miranda R. Polin, Senior Data Analyst at Tandem Diabetes Care in San Diego, California, USA, presented a poster entitled ‘What Happens When People Don’t Bolus for Extended Periods of Time while Using the t:slim X2 with Control-IQ Technology?’ summarising glycaemic data in relation to bolus administration. The study utilised real-world data from users of an automated insulin delivery (AID) system that incorporates continuous glucose monitoring (CGM) and an insulin pump with advanced hybrid closed-loop technology (t:slim X2TM with Control-IQ Technology, Tandem Diabetes Care, San Diego, California, USA) to automate insulin delivery.
Polin provided insight regarding the glycaemic control seen in users of Control-IQ Technology who do not manually bolus for prolonged periods of time. The study demonstrated a higher time-in-range (TIR; 3.9–10.0 mmol/L [70–180 mg/dL]) on the days they did not bolus, compared with manual bolusing, without increasing the risk of hypoglycaemia. Polin suggested that when using systems such as Control-IQ Technology, even without user given boluses, adequate glycaemic control may be achieved under certain conditions. This opens up the possibility that closed-loop technology can positively impact outcomes even when user behaviours are not consistent.
Introduction
Individuals with diabetes, particularly Type 1, often require intensive insulin therapy.1 Polin highlighted the challenges of diabetes management, including monitoring glucose levels, carbohydrate counting, calculating insulin boluses, and administering multiple daily injections, particularly at mealtimes.1 Moreover, the burden of calculating carbohydrates before meals frequently results in missed boluses.2
Technological advancements such as CGM and AID systems have transformed diabetes care,3 with the ADA recommending such devices to achieve early glycaemic control, particularly in Type 1 diabetes.4 Advanced hybrid closed-loop systems, like the t:slim X2 with Control-IQ Technology, combine CGM with an insulin pump and proprietary algorithm to automate insulin delivery.3
This article reviews a study exploring real-world glycaemic outcomes in users who do not manually administer boluses for prolonged periods of time when using closed-loop Control-IQ technology.
Methods
Real-world data from 291,769 users of the t:slim X2 pump with Control-IQ technology (Figure 1) were obtained from the Tandem Diabetes Care t:connect database between January 2020–December 2023.
Figure 1: Interface of continuous glucose monitoring with Control-IQ technology.
Polin explained that the proprietary algorithm aims to prevent hyperglycaemia by predicting CGM levels 30 minutes into the future and pre-emptively increasing basal insulin delivery when glucose levels are predicted to be >8.9 mmol/L (>160 mg/dL). It also pre-emptively delivers an automatic correction bolus up to once an hour based on predicted glucose levels >10 mmol/L (>180 mg/dL) and attenuated to 60%, aimed at achieving a target of 6.1 mmol/L (110 mg/dL; Figure 2). The purpose of this automatic correction bolus is to maximise TIR (3.9–10 mmol/L [70–180 mg/dL]) and mitigate hyperglycaemia that can occur for reasons such as when users do not consistently initiate meal boluses.3
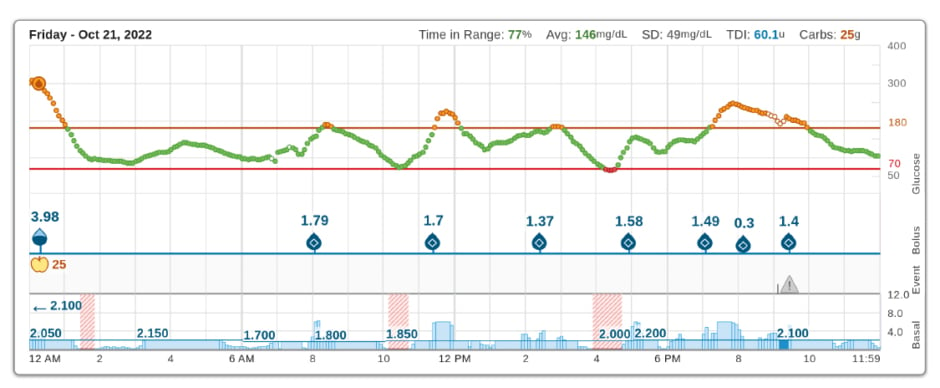
Figure 2: Examples of automatic correction boluses reducing hyperglycaemia in the absence of user-given boluses.
Event: CGM Alerts: 9:07 PM – CGM Out of Range.
Avg: average; Carbs: carbohydrates; SD: standard deviation; TDI: total daily insulin.
Users of Control-IQ technology were included in the analysis if they had used the system for a minimum of 7+ consecutive days without user-initiated bolusing and ≤1 manual bolus per day (referred to as fully closed loop use). Users were categorised into four groups: 7 (1 week), 14 (2 weeks), 30 (1 month), and/or 182 (6 months) consecutive days of fully closed-loop use.
For the individuals who fell into the four categories above, data were analysed including TIR, i.e., the percentage of CGM recordings between 3.9–10 mmol/L (70–180 mg/dL) and time-below-range (<3.9 mmol/L [<70 mg/dL]) on days with fully closed loop use (without bolusing) and compared to days with user-initiated boluses.
Results
The study found approximately one in 10 users (28,790 out of 291,769 users) of Control-IQ technology had sustained periods (7+ days) without bolusing. The database contained 1,728,304 days without boluses, averaging 61 days per user.
Overall, 27,688 users had periods of 7–13 consecutive days without user-initiated boluses, 11,127 users had periods of 14–29 consecutive days, 3,813 users had periods of 30–181 consecutive days (1–6 months), and 102 users had periods of 182 consecutive days or more (6+ months) without user-initiated boluses.
On days without boluses, median TIR was 62% (interquartile range: 50–74%) in all four groups (Figure 3a), whereas median TIR for days with boluses was 57–60% (interquartile range: 46–70%; Figure 3b). The time-below-range was less than 0.6% for both days without boluses and days with boluses.
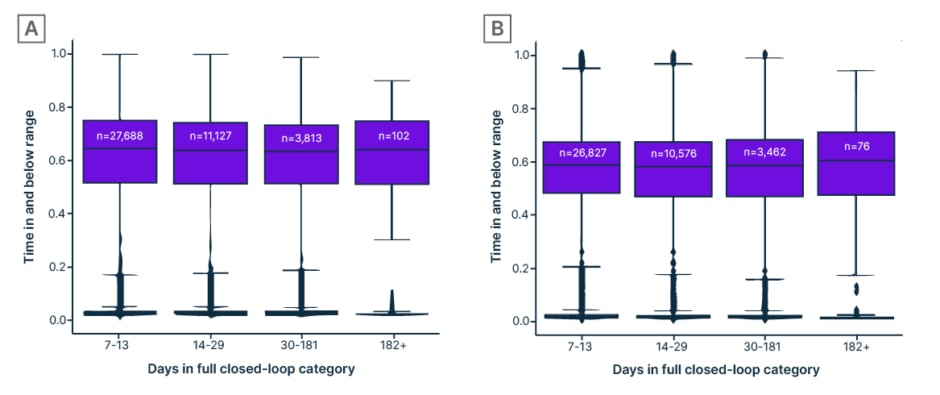
Figure 3: Percentage of time in and below range (3.9–10 mmol/L [70–180 mg/dL]) in Control-IQ technology users who have used the system without boluses for varying periods of time (“days in full closed-loop category”: A) on days with no user-given boluses (fully closed-loop use), compared with B) days with user-given boluses.
Discussion
The study identified that 10% of real-world users of Control-IQ technology have spent 7 days or more without bolusing. Polin reported that the burden of bolusing itself may be a potential reason, though it is not possible to ascertain from these data. Other reasons could include age-related behavioural factors, illness, medications, or changes in circumstances or therapy regimens, including diet control. Another poster presented at the ADA by Fu and colleagues used statistical simulations to demonstrate TIR outcome of 62% with Control-IQ technology when users did not bolus for small-medium carbohydrate meals (≤40 g carbohydrates), which suggests that lower carbohydrate diets may be a good strategy to maximise TIR when not giving boluses.2
One key finding was that glycaemic control in those who do not bolus for extended periods of time have slightly higher TIR (62%) on days when they do not bolus compared to days when they take manual boluses (57%), without an increased risk of hypoglycaemia. The automatic correction bolus is likely useful in counteracting hyperglycaemia on the days without user-given boluses and is a unique feature of Control-IQ technology. Further, the automatic correction boluses do not lead to additional hypoglycaemia, which is a theoretical (but not substantiated) concern. It is unclear why days with user-given boluses had a slightly lower TIR, though it is possible that when people have more hyperglycaemia, they are more inclined to give correction boluses to compensate (reverse causation). Regardless, these data indicate that closed-loop systems like Control-IQ technology may be helpful in maximising TIR, for those who do not, or find it difficult to, bolus consistently. The user and healthcare professional can maximise TIR by programming settings that match their behaviour, such as strengthening the correction factor to maximise the automatic correction bolus for those who do not bolus themselves.3 Polin suggested that Control-IQ technology may attenuate hyperglycaemia enough to be acceptable for glycaemic control in a subset of individuals.
Polin stated that such devices enable ease of use, reduce the need for manual input, and improve accessibility for those who struggle with carbohydrate counting and bolusing. Additionally, Polin noted such technologies have the potential to simplify and reduce the burden of diabetes care by making management more accessible, facilitating individuals in becoming more proficient in device use earlier on, rather than transitioning from one pump to another, ultimately freeing up time and improving quality of life.5
Overall, Control-IQ technology and other systems are valuable in reducing the burden of manual input and improving diabetes management for a wider range of individuals, in line with the ADA recommendations that diabetes technology should be offered to everyone.4
Conclusion
Findings from this study led the authors to recommend that healthcare professionals consider strategies to simplify diabetes management for those using Control-IQ technology, and not require consistent bolusing behaviour as a prerequisite for starting the system. Healthcare professionals can further maximise glycaemic outcomes by optimising settings such as correction factor, discussing low carbohydrate diets, and reviewing additional medications. This approach may reduce the need for stringent bolus behaviours and ultimately reduce the burden of diabetes.







