Abstract
Eosinophilic oesophagitis (EoE) is an emerging disorder that manifests clinically with characteristic symptoms of oesophageal dysfunction and histologically by tissue eosinophilia. This chronic immune-mediated oesophageal disease represents a response primarily to food antigens. The incidence of EoE is escalating in both adults and children. This rise stems not only from heightened recognition but also an increased frequency of allergic/atopic diseases and defective immune tolerance. In adults, EoE presents as intermittent solid-food dysphagia or food impaction, heartburn, and chest pain, typically presenting in young men with known allergies. Presentation differs in children, who experience upper gastrointestinal complaints: abdominal pain, vomiting, feeding difficulties, and/or failure to thrive. Endoscopic features include circular rings, linear furrows, white exudative plaques, strictures, and mucosal fragility. The pathologic hallmark of EoE is mucosal eosinophilia (>15 eosinophils per high-power field) isolated to the oesophagus. Such tissue eosinophilia must be distinguished from gastro-oesophageal acid reflux that responds to optimal proton pump inhibitor (PPI) treatment and from PPI-responsive oesophageal eosinophilia (PPI-ROE). Innovative modalities such as high resolution digitally-enhanced endoscopy and functional luminal impedance planimetry are emerging to better detect EoE and monitor its response to treatment. Current therapeutic strategies involve elimination and elemental diets to avoid food allergens, topical corticosteroids to counter the inflammatory response, and endoscopic dilation of fibrostenotic complications. Other treatments have employed immunosuppressants, antagonists to the leukotriene and T helper Type 2 inflammatory pathways, and biologics that target interleukins, tumour necrosis factor, or immunoglobulin E with variable success. This review highlights the current understanding of the epidemiology, pathogenesis, presentation, treatment, and natural history of EoE, and scrutinises current controversies and future directions for investigation.
INTRODUCTION
Eosinophils are critical effector cells of the innate immune system, particularly when residing in the gastrointestinal tract where they modulate the interplay between foreign antigen exposure and the host immune response. In healthy individuals, eosinophils are normally absent in the oesophagus; their infiltration into the oesophageal epithelium in the context of antigen-mediated inflammatory response is the hallmark of eosinophilic oesophagitis (EoE). Consensus guidelines define EoE as a combination of clinical symptoms related to oesophageal dysfunction and characteristic histopathology: eosinophil-predominant mucosal inflammation (the greatest density being ≥15 eosinophils per high-power field [eos/hpf]). The latter is contingent on excluding secondary aetiologies of oesophageal eosinophilia and persistence of eosinophilia after a trial of proton pump inhibitor (PPI) therapy.1 Recent advances in EoE, such as the development of an animal model, discovery of the EoE transcriptome, and completion of multiple prospective clinical trials have transformed our understanding of this disease.2 Advances in the detection, assessment, andmanagement of EoE aspire to implement the promise of precision medicine.
This review also highlights emerging controversies in the epidemiology, pathogenesis, presentation, treatment, and prognosis of EoE.
EPIDEMIOLOGY
The prevalence of EoE in developed countries is ~0.5%,3 but frequency rates are increasing. In Olmsted County, Minnesota, USA, the incidence of oesophageal eosinophilia increased 27-fold from 0.35/100,000 person-years from 1991–1995, to 9.45/100,000 person-years from 2001–2005.4 Similar trends have been reported in Europe and Australia. Heightened disease awareness however, confounds the interpretation of such temporal trends. Furthermore, the increase in crude EoE incidence may stem from increased oesophageal biopsy rates which in one report rose from 17.0% to 41.3%.5 EoE is identified in 1% of upper endoscopies; this surges to 15% when associated with dysphagia or food bolus impaction, or when upper endoscopies are performed in children.6
Patients with EoE are commonly young adults (20–30 years old), Caucasian males (3:1 sex ratio), often with asthma (30–40%), seasonal allergies (40–50%), and food allergies (10–40%).7 Seasonal and climatic variability in EoE diagnosis adds supporting evidence to the pathogenic role of environmental allergens. Early life exposures associated with EoE include prenatal fever, preterm or caesarean delivery, lack of breastfeeding, and antibiotic use.8 The potential cause of EoE is speculated to be a result of modulating the infant’s microbiome, reducing allergen exposure and subsequently increasing susceptibility to allergic disorders. In this hygiene hypothesis, lack of early childhood exposure to antigens impairs the natural development of immune tolerance and increases the likelihood of developing allergic diseases. The majority (70–80%) of EoE cases occur in men, in contrast to the sex balance observed in other atopic diseases.9 Nevertheless, male and female EoE patients share similar genetic profiles. The preponderance of males with EoE remains unexplained.
HISTOPATHOLOGY
In healthy individuals, the normal oesophagus is devoid of inflammatory cells. EoE is characterised by an infiltration of eosinophils, specifically targeting the oesophagus (Figure 1A). Such changes are often patchy, being distributed along the length of the oesophagus and thus necessitating multiple endoscopic biopsies for a successful diagnosis. The probability of a single biopsy containing >15 eos/hpf is only 63%, whereas four biopsies yield a 98% chance.10 Additional histological changes include a thickened mucosa with basal epithelial cell hyperplasia and papillary lengthening. Eosinophils layer the surface and create microabscesses, a rather characteristic finding in EoE. Dendritic antigen-presenting cells, degranulated mast cells, and CD8+ T cells are also part of the inflammatory process. Thus, isolated tissue eosinophilia may not reflect the total burden of inflammation; ancillary findings such as eosinophilic degranulation should be sought on histopathology. Dilated intracellular spaces, reduced tight junction proteins, and decreased expression of adhesion molecules compromise the mucosal integrity in EoE, fostering a permissive environment for robust antigen presentation and eosinophil trafficking.11 Meanwhile, the extracellular deposition of eosinophilic granule proteins including eosinophilic peroxidases generates oxidative damage. Subsequent collagen deposition in the lamina propria eventually leads to scarring with fibrostenotic strictures and mucosal rings.12 Hence, endoscopic oesophageal biopsies may inadequately capture the depth of disease, missing fibrotic changes in deep layers of the oesophageal wall. Ideally, biopsies should include submucosal sampling to better identify deep eosinophilia and fibrosis (Figures 1B–D).
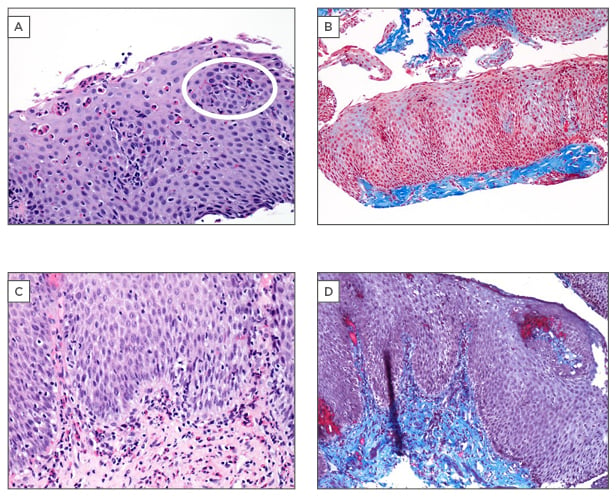
Figure 1: Histopathological features of eosinophilic oesophagitis.
A) Oesophageal mucosal biopsy (x20) demonstrating marked eosinophilic infiltration. The histological hallmark of eosinophilic oesophagitis is >15 eosinophils per high-power field, essential for diagnosis.
The white circle represents an eosinophilic microabscess: a cluster of eosinophils with evidence of degranulation in a prominent surface location. Macroscopically, these eosinophilic microabscesses appear as exudative, white plaques on endoscopy, a feature highly suggestive of eosinophilic oesophagitis and distinct from the tissue eosinophilia from gastro-oesophageal acid reflux. B) Submucosal fibrosis: pathologically deposited collagen stains blue on this Masson’s trichrome stain (x10). Inclusion of the submucosa on oesophageal biopsy allows demonstration of deep fibrosis. C) Presence of abundant eosinophils in deep fibrosed submucosa may be present in eosinophilic oesophagitis, despite relative paucity in superficial mucosal layers (x20). D) Collagen deposition and laminal propria fibrosis demonstrated on Masson’s trichrome strain (x10).
Histological photomicrographs courtesy of Dr Stefan J. Urbanski.
PATHOGENESIS
Familial and Genetic Risks
EoE has been found to display a substantial degree of heritability. The relative risk ratios in family members range from 10–64 depending on the relationship; the ratio is higher for brothers and fathers.13 EoE exhibits an overall sibling risk of 2.4%. This clustering is predominantly driven by a common family environment. Heritability thus has been estimated at 14.5%, whereas a common environment accounts for 81.0%.13 One percent of patients develop EoE in association with an inherited connective tissue disorder (e.g. Loeys–Dietz syndrome, Marfan syndrome Type 2, and Ehlers–Danlos syndrome).14
Numerous genetic associations with EoE have been identified, including: i) overexpression of the eosinophil-specific chemoattractant, CCL26, belonging to the eotaxin-3 chemokine family; ii) variants at chromosome 5q22 associated with overexpression of thymic stromal lymphopoietin precursor (TSLP) that induces the T helper Type 2 (Th2) cytokine response; iii) underexpression of filaggrin, the filament-associated protein that binds to keratin fibres in epithelial cells and serves as a barrier protein; and iv) variants at 2p23 leading to overexpression of calpain 14, an intracellular protease specifically expressed in the oesophagus and upregulated as a function of disease activity.14 A small non-coding mRNA segment (˜1% of the human genome, ˜500 genes) is overexpressed in those with EoE. This ‘EoE transcriptome’ affords insight into disease pathogenesis such as the involvement of eotaxin-3 in eosinophil recruitment and activation, the cytokine milieu, impaired barrier function, and tissue remodelling.14 Such a microRNA genetic signature is conserved in EoE irrespective of sex, age, or atopy and may provide a molecular diagnosis.
Allergen Hypersensitivity
Up to 70% of adult and paediatric patients with EoE have a history of current or previous atopy (eczema, allergic rhinitis, asthma) or other allergies (e.g. urticaria, anaphylaxis). Patients’ response to food elimination diets and the recurrence of EoE with food reintroduction support the pathogenic role of allergen exposure. The primary mechanism of allergen-induced inflammation in EoE appears to be mediated by immunoglobulin (Ig)G4 and not IgE; EoE can develop even in IgE-null mice.15 Not surprisingly, IgE-mediated skin-prick testing in humans identifies only 13% of causative food allergies associated with EoE.16
Oesophageal Dysmotility
Manometric abnormalities are experienced in 60% of EoE patients: these include pan-oesophageal pressurisation, reduced/interrupted peristalsis, hypertensive peristalsis (‘nutcracker’ oesophagus), aperistalsis, and non-specific hypomotility.17 The longitudinal smooth muscle layer, presumably fibrosed, contributes to the weakened and asynchronous contractions.18 Eventually, remodelling and subepithelial fibrosis decrease oesophageal distensibility, as measured by high resolution impedance planimetry. Poor oesophageal distensibility is predictive of dysphagia and food bolus impactions, even without appreciable structural changes on endoscopy.19
Cellular Mechanisms
Eosinophil recruitment to the oesophagus is a key pathogenic event in EoE. Th2-mediated immunity drives this process to upregulate interleukin (IL)-5, IL-13, and eotaxin-3.20 A genetic polymorphism in the TSLP receptor facilitates TSLP upregulation, driving this Th2 response.21 Th2 cells subsequently express IL-5, enabling the differentiation and trafficking of eosinophils to the oesophagus.22 Th2 cells also secrete IL-13 which induces the EoE transcriptome.23 Epithelial exposure to IL-13 increases eotaxin-3 expression; the absence of eotaxin attenuates eosinophil recruitment and protects against EoE.23 Mast cell degranulation also plays a role, releasing transforming growth factor-β, which induces smooth muscle hyperplasia and oesophageal remodelling.24 Chronic damage from eosinophil inflammation leads to collagen deposition in the lamina propria, forming oesophageal strictures.
DIFFERENTIAL DIAGNOSIS
Although eosinophilic infiltration and its persistence after an optimal trial of PPI therapy is the hallmark of EoE, presence of eosinophils is not specific for diagnosis. Rather, it is a histological finding that most commonly reflects allergic or acid-peptic inflammation. The distinction between gastro-oesophageal reflux disease (GORD) and EoE can be challenging due to similar clinical features and frequent co-existence.25 GORD typically has a lower eosinophil density on mucosal biopsy, exhibits acid exposure on ambulatory 24-hour pH monitoring and most importantly, responds to acid reduction with PPI treatment.
Such features however are suggestive rather than conclusive for the diagnosis of GORD. Indeed, in more than one-third of patients with features similar to EoE, the oesophageal symptoms and eosinophilia respond to PPI therapy.26 The tissue eosinophilia disappears, mucosal integrity is restored, and transepithelial allergen flux resolves.27 This entity, PPI-responsive oesophageal eosinophilia (PPI-ROE), may represent a distinct clinical disorder, a subset of primary EoE, or a variant of GORD. The clinical, endoscopic, and histological findings of PPI-ROE are indistinguishable from EoE. Furthermore, the immunologic mechanisms predisposing to EoE are shared in PPI-ROE.28 These comprise features of the Th2 response, IL-13, IL-5, and eotaxin-3 expression, and the EoE transcriptome genetic signature. There may be differential expression of KCNJ2, encoding potassium channels that co-localise with the H1-K1 ATPase proton pump, but our current understanding supports a shared immune-mediated pathogenesis between EoE and PPI-ROE. The precise benefit from PPIs in this context may relate to direct anti-inflammatory effects and restoration of mucosal integrity rather than merely acid suppression.29 The differential diagnosis for oesophageal eosinophilia also includes secondary aetiologies such as hypereosinophilic syndrome, parasitic and fungal infections, Crohn’s disease, allergic vasculitis, leiomyomatosis, and drug-induced eosinophilia.30
CLINICAL AND ENDOSCOPIC FEATURES
Clinical Features
Symptoms of primary EoE vary by age, disease severity, and phenotype. Children present with upper gastrointestinal complaints such as abdominal pain, vomiting, difficulty feeding, food refusal, sleep disturbance, and failure to thrive. Adults are more likely to experience intermittent solid food dysphagia or food impaction, heartburn, and non-cardiac chest pain, typically in young men with atopic diseases such as asthma and allergies.31
Clinical presentation of disease may vary by sex; men typically present with dysphagia and food impaction, while heartburn and chest pain are more commonly reported among women with EoE.32 Food bolus impaction, particularly in young adults, should prompt consideration for the diagnosis: ≤50% of food impaction cases in this population may be attributable to underlying oesophageal eosinophilia.33
Both dysmotility and fibrosis contribute to dysphagia in EoE, which may evolve from being primarily due to intermittent muscular inco-ordination rather than a fixed rigid oesophageal wall, and ultimately mechanical obstruction due to fibrotic stricture. Patients may assume adaptive behaviours to avoid dysphagia: dietary restriction, excessive chewing, or imbibing liquids to prevent solid food obstruction. When symptoms are non-specific, there is often a substantial delay to diagnosis. Regrettably, any delay in effective treatment is complicated by a heightened risk of fibrotic strictures.34 Given the heterogeneity of symptoms, an international expert group developed and validated a symptom-based activity index for adults with EoE, based on a 7-day recall. This EoE activity index quantifies symptoms and behavioural adaptations to dysphagia.35
Endoscopic Features
Endoscopy provides mucosal biopsies for histological assessment, detects complications, and offers therapeutic manoeuvres such as removing food bolus impactions and dilating oesophageal strictures. Most (>80%) patients with EoE have endoscopic abnormalities.36 Classic findings include fixed circular rings (trachealisation), linear furrows, white exudative plaques (correlating histologically with eosinophilic microabscesses), mucosal fragility (‘crêpe-paper’ mucosa), mucosal oedema, strictures, and a narrowed calibre oesophagus (Figure 2). While transient concentric rings are hypothesised to reflect oesophageal muscle contraction, the fixed rings seen in EoE may indicate tissue remodelling from fibrous stricture formation. Individual endoscopic findings are neither sensitive nor specific enough to diagnose EoE. An endoscopic grading system however has been developed to standardise major (fixed rings, exudates, furrows, oedema, strictures) and minor findings (trachealisation, narrow calibre, fragility).37 This standardised reporting tool has good inter-observer reliability and appears valid for accurately identifying EoE patients, predicting histological parameters, and predicting response to therapy.38
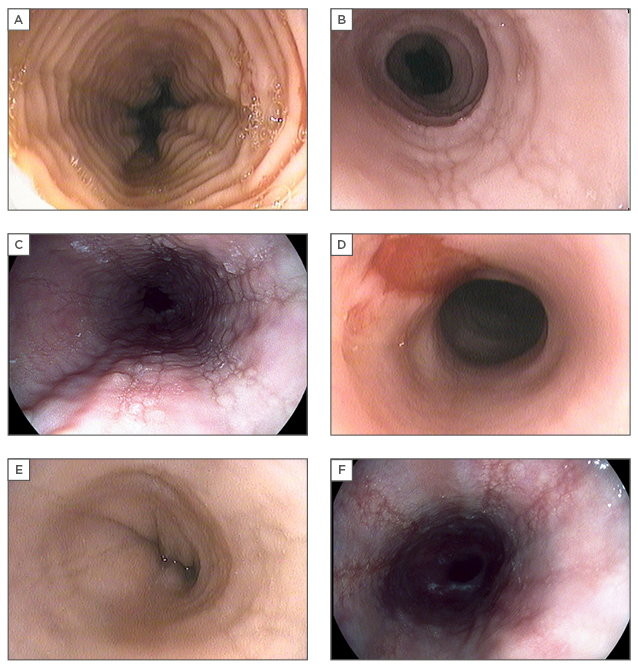
Figure 2: Characteristic endoscopic features of eosinophilic oesophagitis.
A) Fixed circular rings (previously termed trachealisation or feline oesophagus), best appreciated after full insufflation to determine severity (can be mild subtle ridging of mucosa to tight fibrotic bands); B) linear furrows, which run vertically in parallel with the oesophageal axis; C) eosinophilic exudates, representing microabscesses on histology; D) oesophageal tear with ‘crêpe-paper’ highly friable oesophageal mucosa. Superficial tears may be induced by dilation or even with passage of the endoscope; E) oesophageal stricture with narrowing of the luminal calibre; F) longitudinal furrows seen with high resolution digitally-enhanced endoscopy. Digital post-processing per-pixel image enhancement highlights the surface texture, underscoring mucosal abnormalities.
Endoscopic photos courtesy of Dr Paul L. Beck and Dr Marietta Iacucci.
Correlation of Symptoms with Endoscopic and Histological Disease
Although symptoms are frequently used to assess patients with EoE,39 there is a well-recognised clinicopathological dissociation between patient-reported symptoms and objective histological and endoscopic disease activity. For instance, in a large international multicentre cohort study of nearly 270 EoE patients, validated symptom scoring predicted histological and endoscopic remission in fewer than two-thirds of cases.40 Similarly, in randomised trials of topical steroid therapy for EoE, symptomatic response does not always mirror histological endpoints.41 This contrasts with reports in the paediatric literature suggesting that non-specific symptoms such as abdominal pain persist in EoE patients even after achieving histological remission.42 Therefore, physicians should not rely on symptoms alone as a correlate for disease activity.
MANAGEMENT
Treatment Considerations
Recent guidelines have established first-line pharmacological, dietary, and endoscopic treatment options for EoE (Figure 3).1 Certain management strategies warrant further reflection. Firstly, there is no well-defined therapeutic endpoint in EoE. Authors have variably used mean or peak eosinophil density or percentage change in eos/hpf to arbitrarily define histological responses. Even among patients with histologically-inactive disease by eosinophil counts, 50% have ongoing symptoms without resolution of the basal layer hyperplasia.43 In contrast to inflammatory bowel disease, it remains unclear if targeting treatment to complete histological remission alters disease progression. Furthermore, assessing response by histology is limited by the need for repeated endoscopy and patient acceptance. An optimal non-invasive, evidence-based biomarker remains elusive.
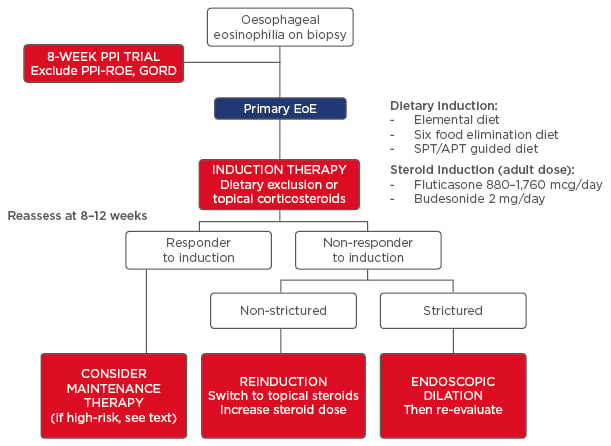
Figure 3: Algorithm for the management of primary eosinophilic oesophagitis.
To exclude GORD or PPI-ROE, first treat with an 8-week course of moderate to high-dose PPI and then repeat the endoscopic and histological assessment. Persistence of oesophageal eosinophilia and exclusion of secondary aetiologies suggests primary EoE. Induction therapy follows with either dietary strategy (six-food elimination diet, SPT or APT-guided diet, or elemental diet) or pharmacological strategy (swallowed topical corticosteroids). After 8–12 weeks of induction therapy, perform a symptomatic, histological, and endoscopic re-evaluation. If the patient does not respond, consider switching treatment strategy and managing fibrostenotic disease complications with endoscopic dilation.
PPI-ROE: proton pump inhibitor-responsive oesophageal eosinophilic oesophagitis; GORD: gastro-oesophageal reflux disease; EoE: eosinophilic oesophagitis; SPT: skin-prick test; APT: atopy patch test.
Secondly, duration of therapy is poorly defined. A limited 8-week treatment regimen suppresses acute inflammation but does not address EoE chronicity.44 There is no conclusive evidence that long-term maintenance therapy alters the natural history of EoE relative to the potential risks from either prolonged corticosteroids or from dietary exclusion, in terms of malnutrition and micronutrient deficiencies. Maintenance treatment should be considered in high-risk patients such as those with long or severe strictures, recurrent food bolus impactions, or debilitating symptoms.45
Finally, patients with PPI-ROE or GORD must be distinguished from primary EoE patients. An 8-week PPI trial followed by endoscopic and histological re-evaluation is recommended to exclude PPI-ROE, especially because >30% of patients with oesophageal eosinophilia will respond to PPIs. Better delineation of PPI-ROE will be achieved with moderate/high dose (omeprazole 40–80 mg daily or equivalent) versus standard dose PPI (omeprazole 20 mg daily).46 GORD must be considered in patients with classic reflux, erosive oesophagitis, or Barrett’s oesophagus. Ambulatory pH monitoring is conditionally recommended in this cohort, although results of pH testing have limited predictive value for treatment response.47
Medical Therapy
Corticosteroids represent the only pharmacological therapy for EoE that improves symptoms and histology. Although systemic steroids were first employed particularly in children, once therapy was tapered, symptoms and tissue eosinophilia recurred. Long-term use was fraught with potential adverse effects. Topical corticosteroids became first-line drugs, achieving histological and symptomatic response rates of 53–95%.48 Aerosolised fluticasone, swallowed from a metered dose inhaler without a spacer, reduces eosinophil infiltration but may not adequately improve symptoms in adults despite histological response.41 Budesonide, best delivered as a slurry mixed into sucralose, syrup, or honey, diminishes oesophageal eosinophilia and improves endoscopic features in the majority of patients.49 This viscous formulation is likely to be more important than the specific corticosteroid.
In addition to reducing eosinophilic infiltration, topical corticosteroids also potentially reverse fibrotic remodelling. It is unclear whether or not this benefit is sustained.50 A 50-week randomised controlled trial of maintenance budesonide found improvements in oesophageal remodelling and mucosal thickness but not long-term symptoms. Unfortunately, this study was limited by sample size and a low budesonide dose (0.25 mcg twice-daily).51 There is no consensus on dosage, formulation, or frequency of maintenance steroid therapy. Short-term topical corticosteroids are safe, though 15% of patients develop oesophageal candidiasis. Concerns about prolonged corticosteroid exposure are related to growth retardation, secondary adrenal insufficiency, and osteoporosis.52
Other pharmacological therapies have been tried with variable success. Montelukast, a leukotriene receptor antagonist that limits eosinophil chemoattraction, has no benefit over placebo.53 Omalizumab (a monoclonal antibody targeting IgE) and infliximab (tumour necrosis factor-α antagonist) also have minimal efficacy.54 More promising are biologic agents specifically targeting key immunopathogenic mechanisms underlying EoE. Mepolizumab and reslizumab (humanised monoclonal antibodies against IL-5) reduce eosinophil and mast cell infiltration, and reverse oesophageal remodelling.55,56 Blocking IL-13 in a small trial reduced eosinophil count and improved barrier function.57 More robust clinical studies are required before these agents join the pharmacological armamentarium for EoE.
Dietary Therapy
Dietary intervention, addressing the allergic pathogenesis of EoE, is effective for inducing histological and symptomatic remission. Achieving similar efficacy to corticosteroids, dietary restriction is often appealing for those patients wishing to avoid pharmacological therapy or at risk for steroid-related complications. Foods can be gradually reintroduced after achieving a response, using subsequent endoscopic and histological evaluation to identify the offending food. The cost and time-intensive nature and challenges with adherence are major drawbacks to dietary strategies.1 It often takes months to identify the right diet, patients may be allergic to multiple foods, and long-term elimination diets pose malnutrition risks.
Exclusive elemental diets are the most effective strategy, decreasing eosinophil density, mast cell content, and basal layer thickness, while improving endoscopic findings and clinical symptoms.58 Such amino acid-based complete liquid formulations are difficult to maintain, especially for adults. Alternatively, the six-food elimination diet empirically removes the most common food allergens: cows’ milk protein, soy, wheat, eggs, peanuts/tree nuts, and seafood. This less restrictive diet reduces tissue eosinophilia and improves symptom scores.59 Targeted elimination diets guided by allergy testing, either as skin-prick or atopy patch-testing, seek to ameliorate the challenge from these broad food restrictions, but produce widely variable results, with response rates as low as 26.6% in adults.60 In adults, elemental diets achieve success in 91%, six-food elimination diets benefit 72%, while allergy test-directed diets improve only 46%.58 Referral to an allergist in this situation should be considered, particularly for children.
Endoscopic Therapy for Eosinophilic Oesophagitis
Endoscopic dilation, using either bougies or through-the-scope balloon dilators, is highly effective (75%) for EoE strictures.61 Multiple dilations are usually required, yet dilation does not alter disease progression. Initially, the reported perforation rates were ≤5% of dilations; frequent deep tears occurred in the friable mucosa (Figure 2D). The perforation rate from recent reports is actually substantially lower, from 0 in an experience of 486 dilations,62 to only 3 perforations (0.3%) in a meta-analysis of 992 dilations.61 Similar perforation rates occur with bougies (0.20%) compared to balloon dilators (0.25%).63 Regardless, a cautious approach to slow step-wise dilation should be undertaken to minimise complications.
NATURAL HISTORY AND PROGNOSIS
A chronic disease, EoE has a poorly elucidated natural history and long-term prognosis. Disease behaviour progresses from childhood through to adulthood, reflecting an evolution in disease phenotype from a predominant inflammatory presentation, manifested by pain and failure to thrive, to a more fibrostenotic phenotype in adults, manifested by dysphagia.64 It is unknown whether this represents a simple progression with diverse manifestations according to age or different diseases. Symptoms and histological eosinophilia persist in nearly all EoE patients, particularly after discontinuation of therapy. Untreated, this inflammation leads to stricture formation and oesophageal narrowing.65 Indeed, an increased duration of symptoms predicts fibrotic complications34 suggesting that untreated tissue eosinophilia is associated with oesophageal remodelling.
Although stricturing, oesophageal narrowing, and food bolus impactions are the most common complications of EoE, spontaneous perforations can rarely occur; ranging in severity from complete Boerhaave’s syndrome rupture to partial or circumferential dissections.66 Secondary perforations from pill ingestion or endoscopic manipulation have also been reported. To date, no malignancies have been associated with EoE; long-term follow-up of this patient population is required. Additionally, complications related to medical treatment of EoE should be considered, including adverse effects of topical steroid therapy (such as growth delay, infection, and adrenal insufficiency), nutrient deficiency from elimination diets, and perforation from endoscopic dilation.67 There is a paucity of validated tools that predict the prognosis of EoE. A key limitation in developing such a tool is the clinicopathological dissociation in EoE, whereby symptomatology poorly reflects histological parameters. Furthermore, complications such as food bolus impaction can occur even in the absence of active eosinophilic infiltration. This highlights the need for comprehensive symptomatic, endoscopic, and histological evaluation in this cohort.40
FUTURE DIRECTIONS
Several technologies are under development to better assess EoE manifestations and predict progression. Advanced endoscopy, including high resolution digitally-enhanced endoscopy with post-processing per pixel image enhancement (Figure 2F), chromoendoscopy, and confocal laser endomicroscopy, all accentuate the mucosal surface texture, highlighting abnormal mucosal morphology and improving sensitivity for detecting subtle changes compared to standard white-light endoscopy. Standard endoscopy only visualises the mucosal surface but inadequately captures the transmural changes in EoE. In contrast, endoscopic ultrasound and quantitative luminal planimetry can assess oesophageal distensibility, mural compliance, and transmural remodelling. To non-invasively monitor EoE activity, use of the microRNA genetic signatures, fractionated exhaled nitric oxide, serum IL-5, eosinophil-derived neurotoxin levels, and string testing to collect oesophageal luminal secretions have been explored. These biomarkers are not yet ready for use in everyday practice. This era of personalised medicine aims to utilise molecular diagnostics to better target therapy. Transcriptome analysis can already distinguish patients likely to respond to corticosteroids. Specific biomarkers presumably should better direct therapy at precise pathogenic events in EoE.14 There has been an explosion of information around EoE over the past two decades. Ongoing innovations will further transform its diagnosis, monitoring, and management in the very near future.








