INTRODUCTION
Data from the National Cancer Institute showed that 50% of myeloma patients survive past 5 years post-diagnosis. This is encouraging but also warns the international myeloma community that there is an unmet need for improvement. Dendritic cells (DC) have been used in clinical trials as cellular mediators for therapeutic vaccination in patients with cancer. There are two main trends: the implementation of next-generation DC vaccines that have improved immunogenicity, and the emerging paradigm of combinations of dendritic cell vaccination with other cancer therapies.1
OBJECTIVES
The Dendritic Cell Myeloma Project in Antwerp, Belgium is part of a set of clinical trials for diseases with unmet medical needs such as acute myeloid leukaemia, mesothelioma, glioblastoma, and multiple sclerosis. The presented study was performed between 2010 and 2013 while the authors were analysing their pilot study of autologous DC vaccination therapy in acute myeloid leukaemia2,3 and before the accessibility in Belgium of monoclonal antibody therapies against CD38 and SLAMF7, and chimeric antigen receptor T cell therapy directed against BCMA.
Methods
In the study, eight myeloma patients were vaccinated, of whom some had high-risk cytogenetics. Median age was 55 years, which is the patient group most in need of better and stronger therapies (Table 1). After leukapheresis, CD14 selection, and 6 days of culturing, white blood cells were uploaded with the WT1 antigen (Figure 1). Vaccination with 10 million DC was performed in the outpatient clinic by intradermal injections near the axillary lymph nodes. Vaccines were given on a fortnightly basis with a minimum of four doses. To monitor haematological responses, bone marrow biopsies were taken before and after four vaccinations. For immune monitoring, delayed type hypersensitivity test after four vaccinations was performed.
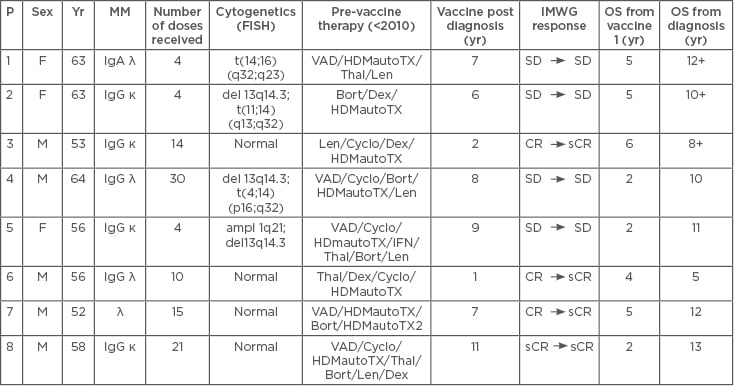
Table 1: Characteristics of patients in the study.
Ampl: Amplification; Bort: bortezomib; CR: complete remission; Cyclo: cyclophosphamide; del: deletion; Dex: dexamethasone; FISH: fluorescence in situ hybridisation; HDMautoTX: high-dose methotrexate + autologous stem cell transplantation; IFN: interferon; IMWG: International Myeloma Working Group; Len: lenalidomide; MM: multiple myeloma; OS: overall survival; P: patient; sCR: stringent complete remission; SD: stable disease; t: translocation; Thal: thalidomide; VAD: vincristine, adriamycine, and dexamethasone; Yr: year
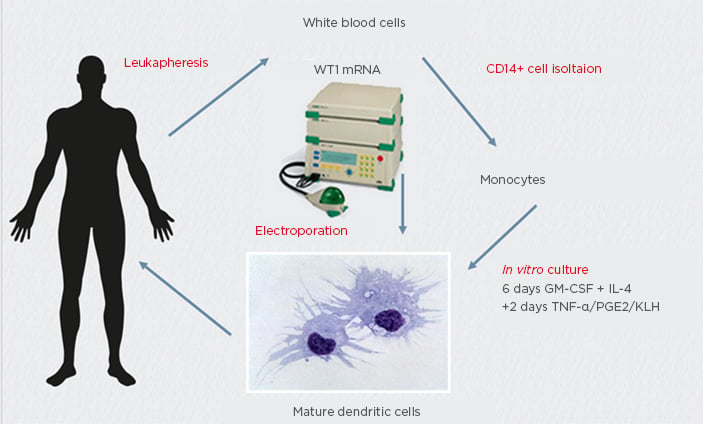
Figure 1: Anti-tumour vaccine manufacturing for multiple myeloma treatment using wild-type mRNA electroporated dendritic cells.
GM-CSF: granulocyte-macrophage colony-stimulating factor; KLH: keyhole limpet haemocyanin; PGE2: prostoglandin E2.
RESULTS
The patients received a median number of 12 vaccinations (range: 4–30). Delayed type hypersensitivity test was positive in all eight patients. International Myeloma Working Group responses after four vaccinations showed a persistent stable disease in four patients, a persistent stringent complete response in one patient, and a conversion from a complete response into a stringent complete response in three patients. Median overall survival was 10.5 years (with a minimum of 5 years and a maximum of 13 years). Three patients are still alive. Median overall survival after the start of vaccination was 4.5 years.
CONCLUSIONS
An induction of DC vaccine-specific T cell immune response was seen in all patients and 5-year overall survival was 100%. With a vaccine production time of 1 week and with total costs of €25,000, DC vaccines are socioeconomically affordable treatment options compared with other immunotherapies. An important issue will be the timing of the vaccination in the whole treatment scheme, especially in the new era of chimeric antigen receptor cell therapy.







