The authors report a prospective Phase II trial to assess (a) if post-transplant cyclophosphamide (PTCy) could be safely incorporated in a timed sequential Bu-Flu regimen, and (b) whether this approach could be used in patients undergoing matched sibling donation (MSD), matched unrelated donation (MUD), and haploidentical haematopoietic cell transplantation.
Patients with hematological malignancies received fixed dose busulfan 80 mg/m2 intravenously (i.v.) either on Days -13 and -12 (n=45), or on Days -20 and -13 (n=10). All patients then received fludarabine 40 mg/m2 i.v. on Days -6 to -2 , followed by busulfan i.v. on Days -6 to -2, and were dosed to achieve target area under the curve (AUC) of 20,000 umoL/min for the whole course based on pharmacokinetic studies. Thiotepa 5 mg/kg i.v. was given on Day -7 to the haploidentical group. Graft-versus-host disease (GVHD) prophylaxis included PTCy 50 mg/kg i.v. given on Days +3 and +4, and tacrolimus starting Days +5. Haploidentical and later MUD recipients also received mycophenolate mofetil. Fifty-five patients were enrolled with a median age of 47 years (range, 15–65).
Diagnoses were acute myeloid leukaemia or MSD (n=30), chronic myeloid leukaemia or MPD (n=9), acute lymphoblastic leukaemia (n=6), lymphoma (n=5), and myeloma (n=5). About half had a haploidentical donor (n=26, 47%), one-third had MUD (n=18, 33%), and the rest had MSD (n=11, 20%). Disease risk index was high in 18 (32%), intermediate in 32 (58%), and low in 5 (9%) patients. Hematopoietic cell transplantation specific comorbidity index was >3 in 22 (40%) patients, and there were no graft failures. The median time to neutrophil engraftment was 17 days (95% CI: 13–39) and that of platelets (> 20K/µL, n=49) was 25 days (95% CI: 11–167). The rate of grade II–IV acute GVHD was 38% (95% CI: 25–51%) and that of grade III–IV acute GVHD was 9% (95% CI: 1–17%) at Day 100. Rates of chronic GVHD and extensive chronic GVHD were 10% (95% CI: 2–28%) and 8% (95% CI: 0–15%), at 1-year respectively. With a median follow up of 17 months (range, 5–28), 1 year overall survival was 71% (95% CI: 60–84%), non-relapse mortality was 20% (95% CI: 9–31%), and relapse rate was 17% (95% CI: 7–27%) (Table 1). One year overall survival in the MSD group was 91% (95% CI: 75–100%); it was 72% (95% CI: 54–96%) in the MUD group, and 62% (95% CI: 45–83%) in the haploidentical group; p=0.11 (Figure 1). Myeloablative-timed sequential busulfan with fludarabine and PTCy is safe in MSD, MUD, and haploidentical HCT. It appears to reduce the incidence of severe acute GVHD and chronic GVHD without an apparent increase in relapse.
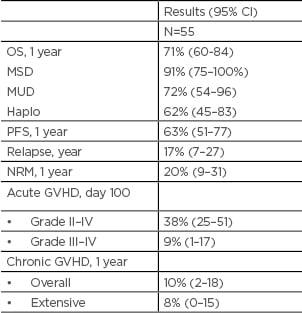
Table 1: Outcomes.
CI: confidence interval; GVHD: graft versus host disease; haplo: haploidentical; NRM: non-relapse mortality; MSD: matched sibling donor; MUD: matched unrelated donor; OS: overall survival, PFS: progression free survival.
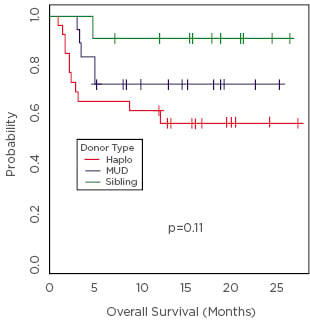
Figure 1: Overall survival.
Haplo: haploidentical; MUD: matched unrelated donation.







