Abstract
Immune thrombocytopenic purpura is a clinical syndrome of thrombocytopenia that manifests as a bleeding tendency, typical skin rashes, easy bruising, or extravasation of blood from the capillaries. Defects in the thrombopoietin-receptor (TPOR)/myeloproliferative leukaemia virus/JAK2 axis leads to haematological diseases such as thrombocytopenia or pancytopenia through the inhibition of the megakaryopoiesis process.
Thrombopoietin-receptor agonists (TPORA), such as eltrombopag, increase platelet count by stimulating the TPOR. Bone marrow (BM) fibrosis has been reported in patients receiving TPORA. Myelofibrosis (MF) may be induced by mutations in JAK2, CALR, and MPL genes. This review gives an insight on MF as a serious side effect induced by eltrombopag.
This review enriches the evidence of MF induced by eltrombopag after long-term administration ranging from 6 months to 7 years. MF is mostly spontaneous and decreases after discontinuation of medication; however, in a few cases it becomes persistent. This major issue should be treated with high concern. The authors recommend that any patient on eltrombopag treatment should be under vigilant observation and closely monitored for any sign of MF by clinical manifestation and any abnormal result from peripheral blood smear examination, and should additionally undergo BM biopsy for confirmation and detection of the severity of MF. The authors recommend discontinuing the medication if this side effect occurs. The authors also recommend to conduct larger studies for longer periods using serial BM before, and periodically after, eltrombopag treatment to evaluate the characteristics of this adverse effect.
INTRODUCTION
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder that lessens the production of platelets.1 Despite treatments being available for ITP, first-line treatments fail in 60–80% of adult patients.2 The initial treatment for ITP is corticosteroid and immunoglobulins (Ig), and splenectomy is then used in cases that fail to respond to steroids. In 2010, the U.S. Food and Drug Administration (FDA) approved anti-RhD Ig as a treatment for ITP patients who are rhesus positive and have not received a splenectomy. Rituximab exerts its therapeutic effect by reducing serum IgG antibody levels and can be used after corticosteroids are tapered and withdrawn. Other second-line treatments include azathioprine, cyclophosphamide, cyclosporine, danazol, mycophenolate mofetil, vincristine, and the novel medication fostamatinib. Recent advances in understanding ITP pathogenesis have highlighted the role of dysfunctional platelet reproduction; this led to a new generation of thrombopoietin (TPO)-receptor agonist (TPORA) therapies, including eltrombopag and romiplostim.3,4
Response rates of these treatments are not universal, and each therapy has its own limitations.2 Myelofibrosis (MF) is a bone marrow (BM) disorder in which the BM is replaced by fibrous tissue, leading to a lack of sufficient blood cells which presents in anaemia, weakness, fatigue, and often, enlargement of the liver and spleen.5,6
Eltrombopag is an effective medication for treatment of ITP; however, the development of BM fibrosis is an immense concern and the authors will spotlight this issue in this scoping review.
METHODOLOGY
Arksey and O’Malley’s methodology was used.7
Identifying the Research Question
This scoping article was conducted to answer the research question: what is known from existing literature about eltrombopag-induced MF in adult ITP patients?
Identifying Relevant Studies
Information Sources and Search Strategy
The search strategy was formulated to identify studies, case reports, meta-analyses, and previous systematic reviews for MF and eltrombopag in adult ITP patients.
This strategy involved searching for research evidence via different sources, including electronic databases like PubMed, Google Scholar, and the Cochran database, as well as reference lists, key journals, relevant organisations such as the American Society of Hematology (ASH), and conference proceedings.
The authors’ method for searching the studies involved three steps. The first step was an initial limited search of a selection of relevant databases, followed by an analysis of text words contained in the title and abstract and of the index terms used to describe the article. A second search using all identified keywords and index terms was then undertaken across all included databases. Thirdly, the reference list of all identified reports and articles were searched for additional studies. Key words that were used included ‘eltrombopag’, ‘myelofibrosis’, ‘immune thrombocytopenia’, and ‘reticulin’. Articles were analysed using Microsoft Excel® as a data extraction tool. Three reviewers implemented the inclusion and exclusion criteria to all citations searched in all databases. Full articles were obtained for those studies that appeared to be relevant. Only the articles that met the inclusion and exclusion criteria were studied.
The inclusion criteria were:
- Human adult studies written in English.
- Studies for chronic ITP adult patients.
- Articles published from 2008 to 2018.
- Any study design (retrospective or prospective).
- Fully published reports, meta-analyses of clinical trials, or reviews.
Any study seemingly meeting the inclusion criteria based on the title, abstract, or both, were put in for closer inspection.
- The exclusion criteria were:
- Trials published in a language other than English.
- Trials that were performed in animals.
- Trials that were performed in children.
- Abstracts.
- Trials outside of the chosen time period (i.e., before 2008 or after 2019).
Study Selection
After an extensive literature search as described, three reviewers applied the inclusion and exclusion criteria to all citations. Full article texts were requested to determine the best article matches with the research question. A deadline was set, after which no more studies could be included in the analysis. The reviewers read the full articles to make the final decision as to whether they should be chosen for inclusion in the review.
Charting the Data
The data was charted and entered into a ‘data charting form’ using Excel. The study-recorded information was filed under the appropriate title headings: author(s), year of publication, title of the study, study location, study details, methodology, and results (Table 1).
Methodology Outcome
The methodology for the study can be seen in Figure 1. The initial search resulted in a total of 201 articles, which included records identified through searching various databases: 161 from Google Scholar, 27 from PubMed, 2 articles related to the Cochran database, and an additional 11 from article reference lists, relevant organisations such as ASH and the FDA, plus conference proceedings that included references identified through other sources.
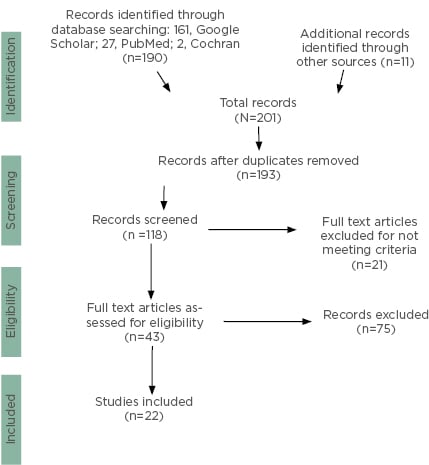
Figure 1: PRISMA flow chart demonstrating literature search and selection of studies.
Eight articles were excluded because of duplications to give 193 total, after which point only articles from 2008 to 2019 were selected and included to give 118 articles.
Abstracts of each of these articles were reviewed and it was determined that many of the articles were not relevant to the topic of this review but more broadly related to cancer and cardiac problems.
The authors examined 43 articles, of which 21 articles were excluded because they were either animal studies, abstracts, or studies performed in children, leaving 22 relevant articles. Of the 22 relevant articles identified, 3 were case reports, 8 were clinical trials, and 11 were reviews, and are summarised in Table 1.8-29
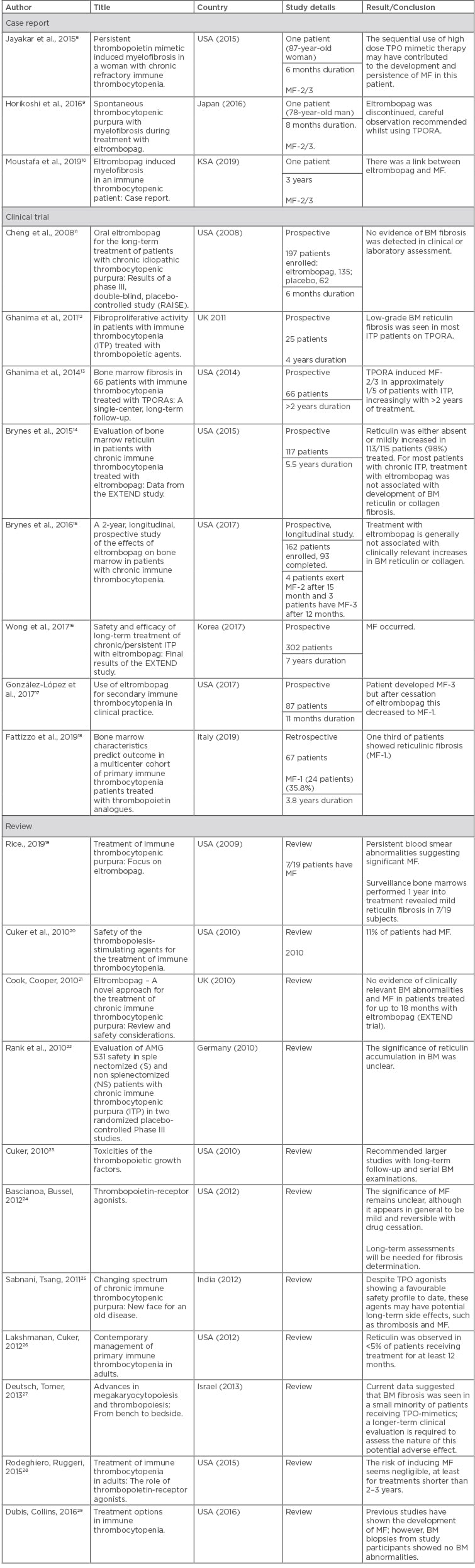
Table 1: Studies from 2008 to 2019 that were included in the literature review of myelofibrosis induced by eltrombopag treatment for immune thrombocytopenia.
BM: bone marrow; ITP: immune thrombocytopenia; KSA: Saudi Arabia; MF: myelofibrosis; TPO: thrombopoietin; TPORA: thrombopoietin receptor agonist; UK: United Kingdom; USA: United States of America.
RESULTS
In the study, three case reports of MF that was induced by eltrombopag were included. MF appeared within 6 months to 3 years following eltrombopag administration, two cases showed persistent MF, and the two others did not. Reports recorded MF with Grade 2/3 according to the World Health Organization (WHO) classification (2008).8,9,30
The case report by Jayakar et al.8 in 2015 presented a case of an 87-year-old woman with chronic ITP who was treated consecutively with two TPO mimetics: romiplostim and then eltrombopag. BM evaluation prior to TPO mimetics treatment showed no evidence of fibrosis. Within 6 months of therapy, the patient developed persistent MF (MF Grade 2/3) that was confirmed by BM biopsy (BMB).8
The case report from 2016 by Horikoshi et al.9 presented the case of a 78-year-old man who had been diagnosed with ITP in 2004. Eltrombopag treatment was initiated in 2014 and 8 months after this the patient underwent a BMB that showed Grade 2 MF.9
The case report by Moustafa et al.10 in 2019 presented the case of a 38-year-old man who was diagnosed in May 2015 with chronic ITP and received eltrombopag as a third-line treatment. The patient was stable for 3 years under eltrombopag, after which time the patient developed a severe reduction in platelet count and failure of eltrombopag treatment. The patient underwent BMB that showed Grade 2 MF. The patient’s pharmaceutical interventions were discontinued but they developed persistent MF.
In the study, eight clinical trials involving a total of 936 patients were reviewed; a number of points of contention regarding the trials were identified and some had limitations. Two prospective trials were completed by Ghanima et al. in 201112 and 201413 which studied a total of 91 patients. These studies confirmed that long-term administration of eltrombopag (2–4 years) caused MF-2/3 and that discontinuation of TPORA may prevent the progression of fibrosis in MF-2/3.12,13 The first trial in 2011 had limitations to the study. Ghanima et al.12 studied 25 ITP patients with TPORA treatment, including 9 patients on eltrombopag, and performed follow-ups on them for 4 years. Eight patients had BMB before treatment in contrast to the other 17 patients. On-treatment biopsies were available in all patients, and five of them were repeated. The results for MF (Grade 1, 2, 3) were significant (19 patients out of 22) but the trial had a small sample size. Some bias appeared in this trial as missing data, particularly related to the pre-treatment BMB for 17 patients. Selection bias appeared in repeating biopsies for patients initially showing extensive fibrosis more than others.
The second trial was done by the same author in 2014 to study 66 patients and provide follow-up for 2–6 years.13 This study carried some limitations as data was missing for some pre-treatment biopsies. Interruption of TPORA for some patients may have affected the degree of fibrosis in the succeeding biopsy, as well as 5 patients having positive grading for MF-1/2 before initiation of treatment.
Another two trials were performed in 201514 and 201715 by Brynes et al., however both had limitations. In the first trial, the authors studied 117 patients treated with eltrombopag for 5.5 years. The study concluded that 2 out of 117 patients had moderate-to-marked reticulin fibrosis MF-2 and MF-3 after 25 months. One patient returned to normal after discontinuing eltrombopag whereas the other patient had persistent MF. Over the 5 years, 98% of patients had findings of MF-0 or MF-1. They concluded that eltrombopag is not associated with MF because reticulin was either absent or mildly increased during treatment and the risk of BM fibrosis may be due to TPORA or the disease.14 No baseline for BMB prior to treatment was identified to be easily compared after treatment.
The second trial from the same author studied 162 patients; 4 patients experienced MF-2 after 15 months and 3 patients had MF-3 after 12 months. From these results, the author concluded that eltrombopag is generally not associated with clinically relevant increases in BM reticulin or collagen.15 However, there is a conflict between his findings and the conclusion. Both of these trials carried limitations as well as attrition bias because of withdrawal of participants caused by MF, personal prerogative, or other adverse effects.
In the RAISE trial conducted in 2008 by Cheng et al.,11 197 patients were studied over 6 months. The study showed that there was no evidence of BM fibrosis detected in clinical or laboratory assessment.11 As a follow-up study, 6 months is a short duration, especially when, in many cases, adverse drug reactions appear after long-term administration.
The study by Wong et al.16 in 2017 studied 302 patients. MF was recorded using the European consensus on grading BM fibrosis and assessment of cellularity. MF-1 was reported in 41% of patients, of which 10 patients (6% of total) had progression to MF-2, and 1 (1% of total) progressed to MF-3. The study carries attrition bias because of withdrawal of some patients or missed data at follow-up. No baseline for BMB pre-treatment was found.
The trial conducted by González-López et al.17 in 2017 studied 87 patients. One patient developed MF-3 after 11 months of being on eltrombopag administration, but after cessation of eltrombopag this decreased to MF-1. Another trial was completed by Fattizzo et al.18 in 2019 which concluded that 1/3 of the patients had MF-1. No baseline for BMB was found before launching eltrombopag. Some patients had received previous treatment, such as romiplostim, that may have caused MF.
In summary, three trials that studied 476 patients, with follow-up periods of 6–66 months, reported that eltrombopag is not associated with MF; however, 6 other trials that studied a total of 547 patients with a follow-up duration from 11 months to 7 years confirmed the association between administration of eltrombopag and development of MF.
Eleven reviews mostly confirmed MF occurrence with long-term eltrombopag administration and gave several recommendations to support this finding such as the implementation of larger studies with long-term follow-up and serial BM examinations. In contrast, three reviews by Cook and Cooper,21 Rank et al.,22 and Bascianoa and Bussel24 concluded that there was no evidence of clinically relevant BM abnormalities or MF with long-term eltrombopag use, and that the significance of reticulin and MF accumulation in BM is unclear.
To summarise, many studies and reviews showed BM changes (increased reticulin and possible BM fibrosis [MF-1/2/3]). Long-term use of eltrombopag may cause changes in the BM starting from 6 months to 7 years.
DISCUSSION
Immune Thrombocytopenia Purpura
ITP was defined as per Francesco Rodeghiero and the panel of International Hematology Working Group’s standardised definitions of primary ITP; an autoimmune disorder characterised by isolated thrombocytopenia (peripheral blood 100×109/L) in the absence of other causes or disorders. The diagnosis of primary ITP remains one of exclusion; no robust clinical or laboratory parameters are currently available to establish ITP diagnosis with accuracy.3 Defects in the TPOR/myeloproliferative leukaemia virus (MPL)/JAK2 axis leads to haematological diseases such as thrombocytopenia or pancytopenia through the inhibition of the megakaryopoiesis process.3,31
Eltrombopag with Immune Thrombocytopenia Purpura
In 2008, the FDA approved eltrombopag (SB-497115, GlaxoSmithKline) for adult patients with chronic ITP.32 The mechanism of action of eltrombopag remains incompletely understood because it could not be studied in preclinical mouse models.31 Eltrombopag induces human megakaryopoiesis, the process leading to platelet production in the blood from the differentiation of BM progenitors to platelet precursors called megakaryocytes (MK). The major cytokine regulating megakaryopoiesis is TPO.31 Eltrombopag selectively binds to the transmembrane domain of the TPOR/MPL oncogene expressed in platelets, MK, and MK-progenitors, that leads to activation of JAK/STAT signalling via STAT5, MAPK, p38, and early response genes. This drives MK proliferation and differentiation and leads to increased platelet production.3,20,33
Eltrombopag may be administered as 30, 50, or 75 mg per day dosages. Eltrombopag increases platelet counts in a dose-dependent manner in patients with relapsed or refractory ITP.34 Eltrombopag may also increase the risk for development or progression of reticulin fibre deposition within the BM. Long-term use of eltrombopag can cause BM changes by showing increased reticulin and BM fibrosis.22 Eltrombopag is an inhibitor of OATP1B1 and BCRP (breast cancer resistance protein) transporters (uptake drug transporters expressed on hepatocytes and other cells). Patients should be closely monitored for signs and symptoms of excessive exposure to the drugs that are substrates of OATP1B1 and BCRP (e.g., rosuvastatin) and consider reduction of their dosages.32 Polyvalent cations (e.g., iron, calcium, aluminium, magnesium, selenium, and zinc) significantly reduce the absorption of eltrombopag; eltrombopag must not be taken within 4 hours of any medications or products containing polyvalent cations such as antacids, dairy products, and mineral supplements.32
Myelofibrosis
MF may be induced by mutations in three key genes: JAK2, which regulates certain enzymes involved in cell growth and the immune response; CALR, whose products are needed for intracellular trafficking; or MPL, which is also important for maintaining cell growth.32 Eltrombopag may increase the risk of development or progression of reticulin fibre deposition within the BM. Long-term use of eltrombopag causes BM changes, shown by increased reticulin and BM fibrosis.35 Eltrombopag has continuous mimetic action on JAK2, MPL, and TPO with long-term administration. This may lead to genetic mutations which cause secondary MF, but more genetic studies are needed in the future to prove this link.
Analysis of Results
Studies suggest increased reticulin deposition in the BM can be caused by TPORA.36 TPORA stimulate reticulin deposition via activation of cytokine production by MK. There are reports of MF with abnormal deposition of collagen and reticulin in BMB of ITP patients treated by TPORA. TPORA-induced MF, however, is described as reversible upon discontinuation of TPO-based therapy in most reports.5,12
Many studies indicated the possibility of BM fibrosis in patients treated with TPORA but the available data about the degree of marrow reticulin fibrosis in ITP patients under treatment with TPORA are still limited. It is clear that certain patients were susceptible to reticulin accumulation in their marrow. The development of MF-2/3 can occur with a duration of treatment from 6 months and above..
The authors believe that degree of fibrosis may depend on specific factors, including dose of medicine, duration of disease, platelet number and immature fraction, splenectomy, and age at time of starting TPORA agent. The degree of fibrosis in the BM is known to be greater with age. More fibrosis also tended to be related to splenectomised patients and a longer duration of disease. Individual susceptibility to reticulin fibrosis in some ITP patients may trigger the development of MF. The mechanism of these findings is currently unclear. Because MF-2/3 was noticed in patients on both romiplostim and eltrombopag, fibrosis is thought to be a class-effect and not related to a specific agent.
Primary MF is a clonal disorder with progressive fibrosis, cytopenias, cellular abnormalities, and altered cytokine release. TPORA-induced fibrosis is a different secondary disease mostly not associated with cytopenia, clonal abnormalities, high lactate dehydrogenase, morphological changes in blood film, and other abnormal biochemical parameters. No clonal abnormalities showed in any of the patients during treatment with TPORA. The authors believe that the exposure time may still be too short to induce clonal abnormalities. For this reason, longer follow-up is recommended to test these agents in this regard as well.
Some other studies have not clearly produced the same results; but this lower proportion of MF-2/3 could be explained by shorter observation times. Also, the duration of ITP before BMB was shorter in these studies. Patients with chronic ITP under TPORA treatment may be on these agents for as long as 5–10 years, meaning observational follow-up is needed to find out whether prolonged exposure to TPORA would lead to relevant grades of MF in many patients. BMB were not performed regularly in all patients, on an annual or biannual basis. In some patients, treatment with TPORA was interrupted before biopsies, which could affect the degree of fibrosis in the specimen taken.
Recommendations During Eltrombopag Treatment
Monitoring patients with the treatment of eltrombopag is a big concern. Haematologists have to monitor any clinical signs of anaemia or thrombocytopenia that may be progressed. Severe fatigue is the most common symptom in MF. Fatigue, fever, night sweats, and severe weight loss are signs of hyper catabolism. Hyperuricaemia can also arise secondary to high cell turnover, in which a high white blood cell count and platelet count may be seen: these cells are needed to compensate the marrow underproduction. Hyperuricaemia leads to gout or renal complications, and routine blood checks are very important to explore anaemia. The differentiation picture of full blood count should be monitored monthly. Peripheral blood smear examination is a useful and simple test to detect MF, and is recommended prior to initiation of therapy to establish the baseline level of cellular morphologic abnormalities and to be monitored monthly. If the blood film includes teardrop-shaped red blood cells and a leucoerythroblastic picture, it may be indicative of MF. The authors suggest monitoring BM reticulin biopsy test for MF confirmation and for the detection of the severity of MF.
CONCLUSION
This review is an addition to the evidence that MF may be induced by eltrombopag after long-term administration, starting from 6 months. MF is mostly spontaneous and normally decreases after discontinuation of medication, but in a few cases becomes persistent. This major issue should be of high concern. Any patient on eltrombopag treatment should be under careful observation and be closely monitored. Routine blood check and peripheral blood smear can be used to detect MF, and BMB will be useful for the confirmation and determination of the severity and staging of the disease. Eltrombopag should be discontinued if this side effect is established. Finally, larger studies for longer periods using serial BMB before and periodically after eltrombopag treatment to evaluate the characteristics of this adverse effect need to be conducted.







