Abstract
Liver fibrosis is a disease that affects patients with hepatitis B virus or hepatitis C virus, harmful alcohol consumption levels, and nonalcoholic fatty liver disease. It is important to assess the cause, disease severity, and prognosis at the time of presentation to determine suitable treatment. The aim of this review article is to outline the recent advances in the diagnosis, management, and treatment of liver fibrosis. A PubMed review was performed encompassing the years 1982–2019 using the following search terms: ‘liver fibrosis’, ‘hepatitis C virus’, ‘hepatitis B virus’, ‘non-alcoholic fatty liver disease’, and ‘alcoholic liver disease’. Results showed that the cornerstone therapy for liver fibrosis is to remove the offending agent and treat the underlying disease. The gold standard method of diagnosis is liver biopsy; however, this procedure is invasive and thus multiple laboratory and radiologic tests are used to help determine the degree of fibrosis. There are few pharmacological agents known to treat fibrosis and they are disease specific. For example, the only proven therapy for fibrosis improvement in alcoholic liver disease is abstinence. The authors concluded that liver fibrosis carries a high morbidity and mortality risk with few therapeutic options depending on the cause and degree of fibrosis. Larger multicentre prospective studies are needed to examine effective agents to prevent, stop, or reduce fibrosis.
INTRODUCTION
Liver fibrosis is the common sequelae of chronic insult to the liver from any aetiology. The most common causes are alcohol-related, fatty liver disease, chronic hepatitis B or C viral infections, autoimmune hepatitis, and metabolic or genetic liver diseases. The disease spectrum of liver fibrosis ranges from non-cirrhotic (stages F0–F3) to cirrhotic (stage F4). Fibrosis is the replacement of tissue with a collagenous scar as a result of repetitive liver insults. Cirrhosis is the end stage of liver fibrosis resulting in regenerative nodular hepatic echotexture surrounded by fibrotic bands and distortion of hepatic vasculature.1,2 Liver fibrosis is a major cause of morbidity and mortality.3 A survey by the Centers for Disease Control and Prevention in 2016 found that there were 4.9 million people living with liver disease.4 Chronic liver disease and cirrhosis is the sixth leading cause of all-cause mortality in people aged 25–64 years.5 Patients may be asymptomatic or present with a wide range of symptoms, including decompensation and liver failure. Liver biopsy is the gold standard for diagnosis; however, many recent advances in biomarkers and imaging are being used as non-invasive methods of diagnosis.6 Rates of fibrosis differ depending on the type of insult, age, and sex.7 Liver fibrosis was previously thought to be a unidirectional process, but many clinical studies have shown that it is a dynamic process with potential for reversibility. The goal of current and future therapies for any chronic liver disease is to prevent, reduce, and reverse the progression of fibrosis to cirrhosis with its complications and the need for liver transplantation.8,9 This review will discuss the current and future advances in the diagnosis, management, and treatment of liver fibrosis.
CLINICAL PRESENTATION
Liver fibrosis often goes unrecognised unless the patient manifests symptoms from complications of cirrhosis. When a patient presents with liver disease, it is important to exclude or confirm cirrhosis, especially when the presentation is with incidental findings of elevated serum aminotransferases, unexplained thrombocytopenia, or abnormal liver imaging. Risk factors for developing liver fibrosis include metabolic syndrome, heavy alcohol consumption, exposure to hepatotoxic substances, and the use of hepatotoxic medications.10 Thus, a careful clinical history and index of suspicion is important to identify the disease early. Physical exam findings that assist with diagnosis include jaundice, spider angioma,11 a nodular liver on palpation,10 splenomegaly, ascites,12 caput medusae, palmar erythema, gynecomastia,13 asterixis,2 and Type 2 diabetes.14 However, many patients are without physical findings and advanced fibrosis is diagnosed by abnormalities on haematological, biochemical, endoscopic, or radiologic evaluation.2,15
PATHOPHYSIOLOGY
Fibrosis is a wound-healing process that becomes dysregulated when repeated insults result in pathologic, chronic fibrinogenesis.16 The common aetiologic agents for chronic repetitive liver damage are harmful alcohol consumption, metabolic syndrome and diabetes, viral infections with hepatitis C virus (HCV) or hepatitis B virus (HBV), toxins and drugs, and autoimmune or metabolic diseases.17,18 All liver cell lines undergo alterations in phenotype due to changes in the microenvironment in the space of Disse.19 The hepatic stellate cell (HSC) is the major driver of hepatic fibrosis followed by portal fibroblasts and bone-marrow derived fibrocytes. Extracellular signals from the innate and adaptive immune systems, such as Kupffer cells, macrophages, natural killer cells, T cells, and B cells, modulate HSC activation, also known as the initiation phase.18,20 In early liver injury, endothelial cells produce a variant of fibronectin that also stimulates HSC activation.19 Hepatocytes stimulate activation through lipid peroxidases leading to oxidative stress, and Kupffer cells stimulate matrix synthesis, cell proliferation, and the release of retinoids by stellate cells.19 HSC release chemokines and cytokines that recruit and activate inflammatory immune cells, contributing to the perpetuation phase of fibrogenesis. In this phase, the HSC proliferate and lead to contractility, fibrogenesis, chemotaxis, matrix degradation, retinoid loss, and cytokine release.18,19 Stellate cell mitogens, such as platelet derived growth factor, endothelin-1, thrombin, fibroblast growth factor, and insulin-like growth factor lead to proliferation. Endothelin-1, along with arginine vasopressin, adrenomedullin, and eicosanoids, activate HSC to increase portal pressures and resistance by constricting sinusoids and contracting the liver. Transforming growth factor B1 is the primary fibrinogenic factor and is upregulated by the transcription factors Sp1 and Zf9. Other factors involved in fibrinogenesis include TNF, lipid peroxides, and acetaldehyde.19 The extracellular matrix, which is made up of molecules such as collagens, glycoproteins, proteoglycans, and glycosaminoglycans, further promotes HSC activation. When the liver becomes fibrotic, the interstitial collagen increases 3–8-fold, a concept known as ‘capillarisation’ that causes destruction of hepatocyte microvilli and endothelial fenestrations.19 As a result, the transport of important solutes to hepatocytes is impaired, leading to hepatic dysfunction.21 Stellate cells are a known source of matrix metalloproteinase-2. Matrix metalloproteinases have been identified as responsive for extracellular matrix remodelling; however, their regulators have not been identified.19 HSC activation and proliferation can be inhibited and even reversed. Mechanisms of reversal involve apoptosis, immune elimination, senescence, and reversion to an inactivated state.20,22 These pathways are promising targets for novel therapeutic agents.
DIAGNOSIS
Diagnosing and assessing the degree of liver fibrosis is important in predicting liver-related morbidity and mortality and the emergence of complications of portal hypertension.23 Histologic scoring systems have been developed to grade (degree of inflammation that reflects ongoing liver disease injury) and stage (amount of current fibrosis) the extent of hepatic disease. The major determinants of inflammatory activity are lymphocytic piecemeal necrosis, lobular necroinflammation, and portal inflammation, which are graded 0–4 in most classification systems. The degree of fibrosis is based on the expansion of fibrotic areas between portal tracts. Stages of fibrosis can range from 0–4 or 0–6 depending on which staging system is used. There are multiple validated scoring systems, including Scheuer/Batts–Ludwig/Tsui which grades on a scale of 0–4; METAVIR, on a scale of 0–4; and Ishak et al.,24 on a scale of 0–6 (Table 1). There are invasive and non-invasive methods of staging for liver fibrosis (Table 2).
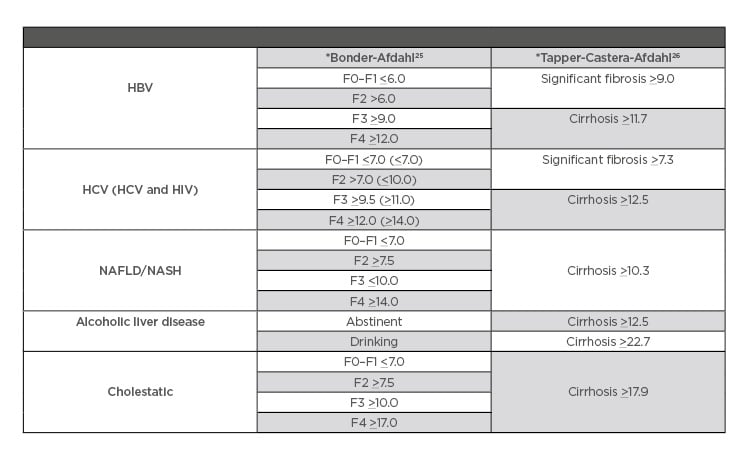
Table 1: Fibroscan evidence-based cut-off references.
*Bonder–Afdahl and Tapper–Castera–Afdahl are names of studies validating different techniques for measuring degree of fibrosis when using Fibroscan.
HBV: hepatitis B virus; HCV: hepatitis C virus; NAFLD: nonalcoholic fatty liver disease; NASH: nonalcoholic fatty steatohepatitis.
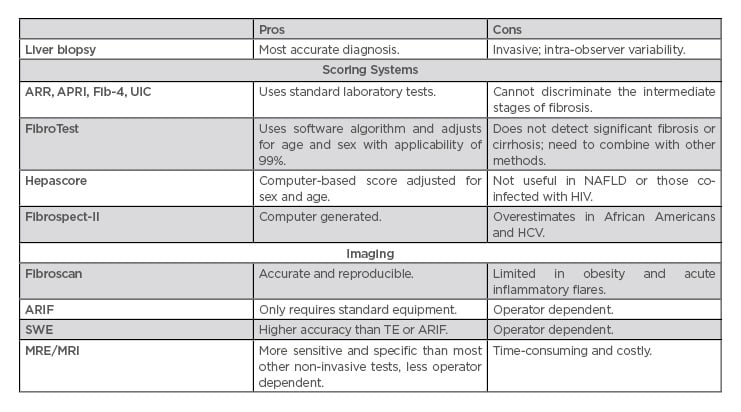
Table 2: Assessing the degree of liver fibrosis.
APRI: aspartate aminotransferase-to-platelet ratio index; ARIF: acoustic radiation force impulse; ARR: alanine aminotransferase ratio; Fib-4: fibrosis-4; HCV: hepatitis C virus; MRE: magnetic resonance enterography; NAFLD: nonalcoholic fatty liver disease; SWE: shear wave elastography; TE: transient elastography; UIC: Universal Index for Cirrhosis.
Combination testing may be a more effective prognostic tool when compared to any individual non-invasive method. In one study, the aspartate aminotransferase (AST)-to-platelet ratio index (APRI), in combination with ultrasound, had a positive predictive value of 80%.27,28
Liver Biopsy
Liver biopsy for diagnosis of cirrhosis is needed when the diagnosis is uncertain based on clinical or biochemical and radiological assessment.10,27 In patients diagnosed with cirrhosis, the liver biopsy is sometimes performed for underlying aetiology of the disease, especially to rule out treatable diseases, such as autoimmune hepatitis. Biopsy can be obtained through a transthoracic, subcostal, or transvenous approach and can assist with diagnosis, prognosis, and management, particularly in those with atypical features or co-existing disorders.23 In a recent prospective study of 176 patients, liver biopsy changed the diagnosis in 55 (31.2%) patients.29 However, there are risks associated with liver biopsy. In a retrospective study by Chi et al.30 there was a 6.00% rate of overall complications, most frequently pain followed by excessive bleeding with an overall risk of death of 0.03%. Absolute contraindications include an uncooperative patient, severe coagulopathy, infection of the hepatic bed, and extrahepatic biliary obstruction. Relative contraindications include ascites, morbid obesity, possible vascular lesion, amyloidosis, and hydatid disease.29
Non-Invasive Measures of Fibrosis
Given the invasiveness and potential for complications with liver biopsy, non-invasive methods to assess the stage of hepatic fibrosis are increasingly being used in clinical practice. The most common modalities include elastography using ultrasound and magnetic resonance technology, as well as measurement of serum biomarkers. Transient elastography (TE), or Fibroscan (Table 1), is an accurate and reproducible method to detect liver fibrosis using ultrasound that can be performed in the outpatient setting. It is also a successful predictor of fibrosis complications such as portal hypertension and hepatocellular carcinoma (HCC).31 The transducer propagates vibrations of low amplitude (50 Hz) to the liver, and the velocity of this propagation is used to determine tissue stiffness. However, the accuracy of TE is limited in obese patients.32 The sensitivity and specificity for the diagnosis of significant fibrosis, advanced fibrosis, and cirrhosis in chronic hepatitis B is 71.6% and 81.6%, 79% and 84.6%, and 80% and 86.6%, respectively, with an overall sensitivity and specificity of 83% and 89%33,34 (Table 1). TE in HCV has an area under the curve of receiver operating characteristic (AUROC) from 0.77–0.90, with a cut-off value of 6.20–8.70 kPa for assessment of significant fibrosis (F≥2); HBV, AUROC, 0.81–0.95; cut off value, 6.30–7.90 kPa; primary biliary cirrhosis (PBC), primary sclerosing cholangitis, and Wilson’s disease, AUROC range is 0.81–0.95 for significant fibrosis.35 TE has also been validated in nonalcoholic fatty liver disease (NAFLD)36 and alcoholic liver disease (ALD).37 However, TE overestimates the degree of fibrosis in the setting of inflammatory activity. Thus, if a patient is in an acute flare, it is recommended to wait until alanine aminotransferase (ALT) levels have stabilised.35
Acoustic radiation force impulse (ARIF) of the liver is an additional ultrasonographic method to measure liver fibrosis, with a sensitivity and specificity of 84% and 92%, respectively, and only requires standard ultrasound equipment. However, it is operator dependent.34,38
2D-shear wave elastography (SWE) is a real-time technique that produces a colour-coded image from radiation generated by an amplitude modulated beam of focussed ultrasound.39 A recent meta-analysis proposed that SWE may be an equally helpful method for detecting liver fibrosis and may have higher accuracy than TE and ARIF at detecting fibrosis severity.39 The pooled sensitivity and specificity for the varying stages of fibrosis are 85% and 81% for F2 or greater, 90% and 81% for F3 or greater, and 87% and 88% for F4 or greater.40 However, this method is also operator dependent. As with ARIF, the operator has the potential to influence the findings based on where they place the region of interest, as opposed to TE where this variability in operator technique is not present.41
Magnetic resonance elastography is a contrast phase study that uses mechanical wave propagation to assess tissue stiffness and can also be used to assess portal hypertension and spleen stiffness simultaneously. The sensitivity in chronic hepatitis B in significant fibrosis, advance fibrosis, and cirrhosis were 92.8% and 93.7%, 89.6% and 93.2%, and 89.5% and 92%, respectively.33 Overall sensitivity and specificity is 100% and 96%, respectively.34 Although more sensitive and specific than the other non-invasive tests with less operator-variability, this method is more time-consuming and costly than the other imaging modalities.42
Serological Markers
These biomarkers for assessing fibrosis stage can be based on tests specifically used for this purpose or tests needed for standard of care.
Markers Based on Standard of Care Laboratory Parameters
Markers based on standard of care include many scoring systems, such as aminotransferase-to-ALT ratio (ARR),43 APRI,44 and fibrosis-4 (FIB-4).45 One of the most commonly used formulas, APRI, is calculated using the patient’s AST level, corrected for the upper limit of normal, and platelet count. When combining serum ferritin (SF) with the AAR, APRI, FIB-4, and Fibro-Q, SF plus APRI was the most reliable to predict cirrhosis.43 On the other hand, the Universal Index for Cirrhosis (UIC) had the highest AUROC when compared to Fibro-Q, FIB4, APRI, and ARR, and can be used in all types of fibrosis.46 Fibro-mark was found to be a superior predictor of fibrosis over existing scores in those with chronic HCV.47 The NAFLD fibrosis score uses routine demographic and laboratory variables, such as age, glucose level, BMI, platelet count, albumin, and AST/ALT to differentiate those with advanced fibrosis with an AUROC of 0.88 and 0.82.48 In addition, the BARD score is able to determine advanced fibrosis at stages F3 and F4, with a negative predictive value of 97%.49 While accurate in excluding or confirming significant fibrosis, these formulas often fail to discriminate the intermediate stages of fibrosis necessitating the use of other non-invasive methods.
Markers Requiring Special Tests Outside Standard of Care Laboratory Parameters
FibroTest is a clinically validated measure of fibrosis that analyses serum biomarkers (α2-macroglobulin, apolipoprotein A1, haptoglobin, gamma-glutamyl-transpeptidase, and total bilirubin) and uses a software algorithm to determine an individual score while adjusting for age and sex at a mean applicability rate of 99.03%.50 However, it is limited in detecting significant fibrosis and cirrhosis and thus it is recommended to combine with other methods of diagnosis to improve accuracy.51
Hepascore is another computed-based fibrosis score adjusted for sex and age that analyses serum levels of total bilirubin, gamma-glutamyl transferase, α2-macroglobulin, and serum hyaluronic acid (HA). This test has been used as a primary screening method to determine the need for liver biopsy due to its ability to predict the level of fibrosis, particularly cirrhosis.52 Hepascore has shown better diagnostic predictability in HCV, HBV, and ALD than for NAFLD and those co-infected with HIV.53
FIBROspect-II (FS-II) uses α-2 macroglobulin, HA, and tissue inhibitor metalloproteinase type 1 to estimate liver fibrosis.54 However, FS-II may overestimate degree of fibrosis in African Americans with HCV.55 In one study, HA was equally as effective at determining the stage of fibrosis in HCV as compared with the FS-II score and, thus, may be a more cost-effective alternative for screening.56
Enhanced liver fibrosis score uses procollagen III amino terminal peptide, HA, and tissue inhibitor of metalloproteinase I and can allow for the avoidance of liver biopsy in approximately 60% of patients.57
MANAGEMENT (TABLE 3)
Table 3 summarises effective therapies for the reduction of liver fibrosis in patients with various diseases.
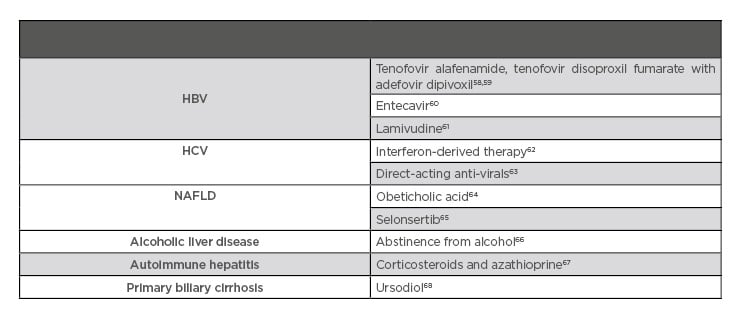
Table 3: Therapy proven to aid in fibrosis regression.
HBV: hepatitis B virus; HCV: hepatitis C virus; NAFLD: nonalcoholic fatty liver disease.
General Management
Preventive hepatology focusses on nutrition; promoting a healthy lifestyle, including exercise and abstinence from alcohol consumption; vaccinations; and screening for HCC. Malnutrition is a frequent complication in chronic liver disease and, along with obesity and sarcopenia, can lead to a worse prognosis.69 Dietary interventions should be individualised and may focus on nutritional micronutrient replacement, adequate protein calorie intake of 1.2–1.5 g/kg daily, and low sodium consumption. Common micronutrient deficiencies include thiamine, B12, folic acid, retinol, vitamin K, vitamin D, zinc, selenium, and magnesium. Patients should consume 5–7 small meals per day to prevent consumption of too much protein in a single meal. Some may benefit from a late-night snack due to the evidence supporting improvement in sarcopenia and quality of life.70 In patients that are overweight, weight loss has been proven to not only improve ALT/AST and insulin resistance but also quality of life in ALD and HCV liver disease patients.71 Recommended vaccinations, other than those recommended for the general population, include hepatitis A and B and pneumococcal vaccinations, regardless of age.72 Screening for oesophageal varices begins once the diagnosis of cirrhosis is made.73 HCC screening is also typically performed once a patient has developed cirrhosis; however, there is increasing evidence to support the need for screening in those with earlier stages of fibrosis. In recent studies, the incidence of HCC in those without cirrhosis was found to be elevated in those with HBV, NAFLD, and metabolic syndrome.74,75 However, those with F3 fibrosis have much lower cost-effectiveness for screening, as well as a decreased risk for development of liver disease complications and better survival than patients with cirrhosis (F4 fibrosis).76 Portal hypertension is a complication of advanced liver fibrosis that can result in variceal bleeding and ascites. Once a patient develops cirrhosis, a variceal screening oesophagogastroduodenoscopy should be performed and repeated after 1–3 years, depending on findings.77
The most effective way to manage hepatic fibrosis is to eliminate the stimulus or harmful cause of hepatic damage, but this is not always feasible. No anti-fibrotic agents have been approved for human use that work effectively at eliminating or reducing fibrosis in the clinical setting. Due to the disease complexity, it is suspected that combination therapy may be needed to target two pathways to effectively treat fibrosis and cirrhosis.78 The current mainstay of treatment for liver fibrosis is to treat the underlying disease.8
Hepatitis B Virus Infection
Long term suppression of chronic HBV can lead to regression of fibrosis and cirrhosis. Tenofovir disoproxil fumarate (TDF) is a prodrug of tenofovir and, when compared to adefovir dipivoxil as single therapy for chronic HBV, demonstrated a significantly greater number of complete responders at 48 weeks, defined as HBV DNA <400 copies/mL and histological improvement (reduction of ≥2 points in Knodell necroinflammatory score).79 In a study of patients randomised to TDF with adefovir dipivoxil, 87% had histological improvement and 51% had regression of fibrosis at Week 240 (p<0.0001). In addition, of the 96 patients with cirrhosis, 74% no longer had cirrhosis and only 3 of 252 patients progressed to cirrhosis at 5 years (p<0.0001).58 Both nucleoside-naïve patients who were treated with entecavir60 and patients treated with lamivudine therapy61 had significant histological improvement and regression of fibrosis or cirrhosis. Furthermore, anti-viral therapy significantly improves decompensated cirrhosis, as well as liver function and mortality rates.80 Tenofovir alafenamide is a prodrug that was developed to allow more efficient delivery of the active metabolite than TDF and had greater reductions in FibroTest scores at 48 weeks (mean change 0.07 versus 0.04; p=0.007).59 It is important to continue HCC screening because HCC in serologically cured HBV can occur in those with pre-cirrhosis or cirrhosis.81 However, those with F3 fibrosis have much lower cost-effectiveness for screening as well as decreased risk for development of liver disease complications and better survival than patients with cirrhosis (F4 fibrosis).76
Hepatitis C Virus Infection
Historically, HCV infection was treated with interferon and ribavirin. Interferon-derived therapy resulted in a 50% regression in cirrhosis in the 30% who achieved a sustained virologic response (SVR). However, in those with advanced cirrhosis, only 5% saw regression of their liver disease over a 10 year period.62 Lower baseline stage of fibrosis, sustained viral response, age <40 years, BMI <27, and viral load <3.5 million copies per mL were independently associated with regression of fibrosis after treatment.82
The newer direct-acting antivirals (DAA) may eradicate HCV, but have not yet been proven to improve survival and complications.83 In a prospective study of 70 patients, 48.6% had a >30% improvement in vibration-controlled TE.74 In another prospective study of 304 patients, TE was used to assess the degree of fibrosis after DAA therapy and showed that 65.1% achieved at least a 20% reduction in liver stiffness.84 Another study of 260 patients on DAA showed a significant fibrosis regression in 40% with baseline advanced fibrosis versus mild fibrosis (52.3 versus 22.5%; p<0.001).85 Larger prospective trials are needed to further confirm these results. Furthermore, it is difficult to determine their effect on regression of fibrosis and cirrhosis because liver biopsy is not commonly used. TE may overestimate the degree of regression of disease and is thought to falsely show lower fibrosis staging due to decreased inflammation once the virus is cleared.63,86 Data matching TE and liver biopsy after SVR is lacking and it is unclear how long a patient will need monitoring after SVR. As with HBV clearance, those with HCV clearance will also need follow-up for HCC.87 Some patients with HCV were even seen to have an unexpected high recurrence rate of HCC at 27–29% after treatment with ablation or radiation. This study suggests this population receiving DAA may need closer screening.88 However, in the age of DAA, a reduction of 30-50% was seen in those with HCV requiring wait-listing and subsequently liver transplant, indicating a tremendous success with these medications. Furthermore, there are approximately 600 donor livers each year now being allocated to other forms of chronic liver disease.89,90
Nonalcoholic Fatty Liver Disease
The only proven treatment for NAFLD is lifestyle modification, including control of the components of metabolic syndrome. Thus, therapy is directed at controlling risk factors such as insulin resistance, decreasing delivery of fatty acids to the liver, and the use of hepatoprotective medications.91 Weight loss improves histologic features of NAFLD, particularly nonalcoholic fatty steatohepatitis (NASH). The highest rate of their reduction is seen in those who lose >10% of bodyweight, with 90% resolution of NASH and 45% regression of fibrosis.92 In a recent study, a text messaging approach encouraging a healthy lifestyle improved weight loss and hepatic function tests in patients with NAFLD.92,93 In another study, some patients with only 3.0–4.9% weight loss achieved remission of NAFLD at 12 months.94 Previously, NASH was thought to increase the risk of adverse outcomes, but, in a randomised retrospective study of 646 biopsy-proven patients with NAFLD, the stage of fibrosis rather than NASH was determined to predict adverse related events.95 Furthermore, it is suggested that fibrosis stage should be part of predicting all-cause mortality secondary to cardiovascular disease and development of chronic kidney disease.96,97
There is currently no U.S. Food and Drug Administration (FDA) approved medication for the treatment of NAFLD or NASH, but multiple trials are underway. A meta-analysis of thiazolidinediones in the treatment of NASH showed significant histological improvement in ballooning degeneration, lobular inflammation, and steatosis, although this is at the expense of significant weight gain.98 A Bayesian network meta-analysis found that thiazolidinediones, vitamin E, pentoxifylline, and obeticholic acid (OCA) improve ballooning degeneration, lobular inflammation, and steatosis, while only pentoxifylline and OCA improve fibrosis.64 A Phase III, randomised, double-blind, placebo-controlled trial (REGENERATE)99 is being conducted to assess the benefits of OCA in patients with NASH and advanced fibrosis. In the Phase IIb FLINT trial,100 OCA demonstrated superiority over placebo based on an intention-to-treat (p=0.0002) in addition to improving liver fibrosis (p=0.004) in NASH and was well tolerated.65 AURORA is a Phase III, randomised, double-blind, placebo-controlled study on cenicriviroc for the treatment of liver fibrosis for those with NASH.101 RESOLVE-IT is a Phase III multicentre study looking at the effects of elafibranor in patients with NASH and fibrosis.102 Selonsertib was studied in a Phase II trial103 with NASH patients and was determined to be superior to placebo in improvement of one stage of fibrosis or greater, and improved fibrosis without worsening NASH.65 STELLAR 3104 and STELLAR 4105 are Phase III studies examining selonsertib in those with NASH F3 and compensated F4 fibrosis, respectively. ATLAS106 is a Phase II study examining selonsertib, firsocostat, and cilofexor, both individually and in combinations, in patients with bridging fibrosis or NASH; the results of this study have thus far been promising, with minimal side effects and a reduction of 30% measured hepatic fat based upon MRI.
Alcoholic Liver Disease
The mainstay of treatment for ALD is a reduction in alcohol use.66 Abstinence can lead to total resolution of hepatic steatosis with the most benefits seen in patients with jaundice or ascites. Abstainers’ probability of survival was found to be 87% compared to 55% in persistent drinkers.107 A recent study suggested in those with NAFLD, even moderate alcohol consumption (10.0–29.9 g per day for men and 10.0–19.9 g per day for women) can result in worsening fibrosis.108 Corticosteroids have been studied in both ALD and alcoholic hepatitis (AH), although results are variable. Prednisolone studied in the STOPAH trial did not show a mortality benefit at 90 days or at 1 year in patients with severe AH.109 However, the American Association for the Study of Liver Diseases (AASLD) and the American College of Gastroenterology (ACG) recommend a trial of steroids in patients with severe AH because of the trend for 28-day mortality benefit among STOPAH participants in the prednisolone therapy arm.66,110 Oxidative stress is an important component in the pathology of ALD; however, antioxidants like s-adenosyl-L-methionine, vitamin E, and silymarin have failed to show efficacy in the treatment of ALD.111
A Phase II study of livercellgram,112 a stem cell therapy, is being conducted in patients with ALD. Many ongoing clinical trials for ALD are focussed on targeting the gut–liver axis (probiotics, antibiotics, zinc), inflammation and oxidative stress (anakinra, extracorporeal cellular therapy, ASK-1 inhibitor selonsertib, and metadoxine), and regenerative agents (G-CSF and IL-22).110,113-120 There is a growing interest in investigating the use of probiotics in ALD due to its close association with gut microbial alterations; however, the precise mechanism needs further investigation.121 A current Phase II trial122 is investigating rifaximin in ALD. The remainder of clinical trials focus on AH. Selonsertib (ASK-1 inhibitor) has completed a Phase II trial123 with prednisolone versus selonsertib alone in those with AH and data is currently pending. Metadoxine showed improvement in 3 and 6-month mortality in those with severe AH, aided with alcohol abstinence.124 A Phase IV trial125 is underway investigating the efficacy of G-CSF in patients with severe AH. IL-22 is overexpressed in liver regeneration and repair, and a current Phase II trial126 is underway evaluating the use of IL-22 in AH.
Autoimmune Hepatitis
Corticosteroids and anti-inflammatory agents are the mainstay of treatment for autoimmune hepatitis. In those with mild disease, a low dose of prednisone may be used, such as 30 mg daily. In those with more severe hepatitis, the recommendation is to begin with a higher dose of 60 mg prednisone daily. If patients are at increased risk of side effects (brittle diabetes, post-menopausal women, hypertension, emotional liability, obesity, or osteoporosis), a low dose of prednisone (30 mg daily) combined with azathioprine (50 mg daily) is used for initial treatment. If a patient has significant cytopenia, is pregnant, or has a malignancy, azathioprine should be avoided. Corticosteroids have been shown to improve or stabilise fibrosis in about two-thirds of patients.67 If continued on steroids, patients should be monitored closely with annual bone densitometry and should also receive hepatitis A and B virus vaccinations, regardless of age.127
Primary Biliary Cirrhosis
Although PBC is thought to be a form of autoimmune disease, immunosuppressive therapy has not proven beneficial in this population. Ursodeoxycholic acid (UDCA) is the mainstay of treatment and has been shown to delay the time of the liver transplant and death in patients with PBC.128 Although UDCA effectively decreases AST and ALT, it did not appear to improve existing cholestasis or fibrosis compared to placebo in one study.129 However, in another study, UDCA was found to significantly delay the progression of fibrosis in PBC with 76% on UDCA remaining in early stage disease versus 29% in the placebo group.68 Furthermore, the decreased need for ALT among all aetiologies for PBC treated with UDCA support its use.130
OCA showed benefits as monotherapy or in conjunction with UDCA over placebo in patients with PBC in single centre studies.131 COBALT, a Phase IV, double-blinded, randomised, placebo-controlled multicentre trial study is being conducted to further assess OCA in PBC.132 As HSC are the main drivers of liver fibrosis, they remain an important potential target for therapy. Although many drugs used in mouse models show improvement in liver fibrosis, medications targeting HSC have yet to be approved for the treatment of liver fibrosis.133
SUMMARY
Common causes of hepatic fibrosis are chronic viral infection with HBV or HCV, harmful alcohol consumption, and NAFLD. With accelerating obesity rates worldwide and effective cure of HCV with DAA, alcohol and NAFLD are emerging as the leading causes of hepatic fibrosis and cirrhosis, with its related complications. Apart from treatment of the underlying aetiology and risk factors, several new therapeutic approaches are being studied with potential to prevent, stop, or reverse the progression of liver fibrosis. The field is advancing rapidly, especially in NAFLD, as many studies have consistently shown that fibrosis stage, and not fatty liver or inflammation severity, determines the long-term outcomes including hepatic and extra-hepatic outcomes.








