Abstract
Transcatheter heart valve retrograde migration after transcatheter aortic valve implantation is unusual. It can occur during the implantation of the aortic transcatheter heart valve, i.e., intra-procedural, or in the first few days following the intervention. Transcatheter valve embolisation and migration soon after deployment typically results from the implantation of a prothesis that was undersized for the annulus, an unreasonably low implantation, or the expulsion of the device following deployment by an excessive ventricular contraction. The presented case highlights the importance of the timing of the complication that has taken place, in this case, intra-procedural, which has become relevant to the research.
INTRODUCTION
Transcatheter valve embolisation and migration (TVEM) into the left ventricle (LV) is especially significant and requires conversion to an emergency surgical technique, with unusually high mortality reported.1 TVEM may be responsible for extreme acute aortic regurgitation and cardiogenic shock. Although transcatheter aortic valve replacement (TAVR) is less invasive than a surgical aortic valve replacement, periprocedural complications remain common. Among these is an uncommon but serious complication of prosthesis displacement into the LV, requiring emergency cardiac operation related to high mortality rates.2
The authors present a rare case of intra-procedural retrograde migration of the transcatheter heart valve (THV), potentially linked to low implantation in a native aortic valve with extreme asymmetric calcification, which presented as a sudden-onset, electro-mechanical dissociation leading to fatal cardiovascular collapse.
CASE REPORT
A 77-year-old female was admitted with complaints of progressive breathlessness on exertion, New York Heart Association (NYHA) Class III. She had experienced an anterior wall myocardial infarction in 2008 and underwent percutaneous coronary intervention to the left anterior descending artery (LAD). Clinical and echocardiographic examination confirmed the presence of severe aortic stenosis and calculated a logistic Euro SCORE of 26. The patient was frail; her Society of Thoracic Surgeons (STS) risk score was 5.3%.
She was referred to the institutional heart team, who considered her for TAVR as a first option. Her echocardiogram showed fair LV function (ejection fraction 55%) with hypokinesia of the distal interventricular septum and apical segments, severe calcified aortic stenosis with a valve area of 0.6 cm2, and a peak/mean gradient of 88/55 mmHg.
Coronary angiography revealed a patent LAD stent with non-obstructive plaques in the LAD and right coronary artery. A multidetector CT scan revealed diffuse atherosclerotic changes in the ascending aorta particularly; there was dense calcium at the sino-tubular (ST) junction. The CT-derived measurements of the ascending aorta and aortic annulus measured a mean aortic annulus diameter of 21 mm, perimeter of 66.9 mm, and annulus area of 322.5 cm2. The left coronary ostial height was 11.4 mm and the right coronary ostial height was 13.2 mm (Figures 1A, B). The size of both external iliac arteries was borderline for the balloon-expandable THV; the right external iliac artery was smaller than the left. Hence left femoral artery approach was preferred, particularly as the right femoral artery also had moderate tortuosity with mild stenosis (Figure 1C).
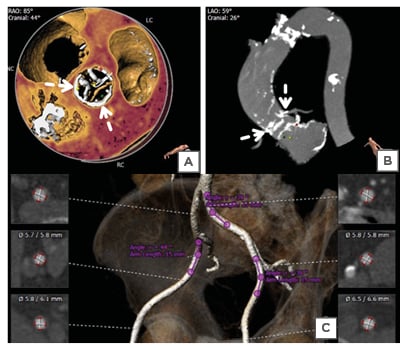
Figure 1: Computed tomography of the ascending aorta. A, B) 3D reconstruction obtained with 3mensio software (Pie Medical Imaging, Maastricht, Netherlands) showed the presence of a thick, unequal calcified arc (>180°) at the sino-tubular junction (dashed arrows) and annular calcium extending to the left ventricular outflow tract. C) 3D reconstructed volume-rendered image of the iliofemoral arterial system revealed that the right common iliac artery had mild stenosis and was mildly tortuous. The left iliac artery had less tortuosity without major stenosis.
The trans-femoral TAVR under general anaesthesia via a left femoral artery approach proceeded under the fluoroscopic and transthoracic echocardiographic guidance (Figures 2A, B). A 14 French (Fr) expandable Python introducer sheath (Myval, Meril Lifesciences Pvt. Ltd, Vapi, India) could traverse without any resistance (Figure 2C). A balloon aortic valvuloplasty was performed using an 18×40 mm balloon under rapid right ventricular pacing at 180 beats/minute (Figures 2D, E). Balloon-expandable THV (Myval, Meril Lifesciences Pvt. Ltd, Vapi, India) could not cross the left femoral artery despite hard push (Figure 2F). The authors then took the introducer sheath outside and attempted to dilate it with larger 16 Fr sheath dilatators; they then tried balloon dilatation of the inline sheath to increase the internal diameter of the sheath and allow the balloon THV to pass through. Also considered was direct vascular access via arteriotomy for insertion, but this was ruled out due to the extensive surgical dissection. Before the closure with Proglide™ devices (Abbott Vascular, Redwood City, California, USA), a repeat angiography was performed to rule out any vascular complications.
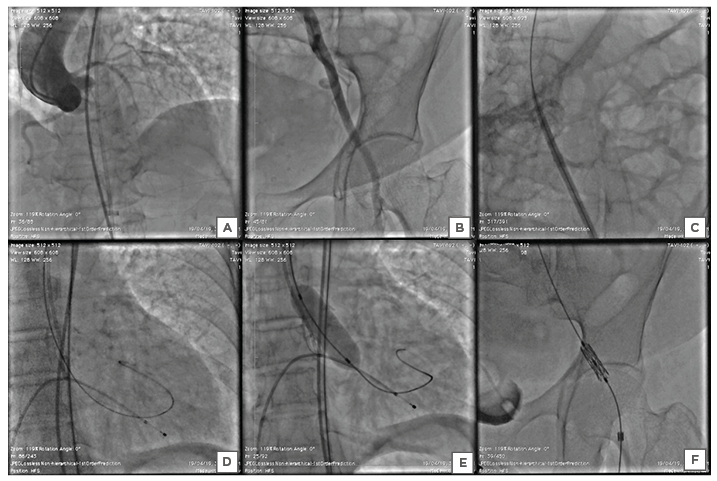
Figure 2: Stepwise procedure of the trans-femoral transcatheter aortic valve implantation. A) Baseline aortic root angiography demonstrated a calcified aortic valve with mild aortic regurgitation, and visualisation of the ascending, arch, and descending thoracic aorta and coronary arteries. B) The pigtail was inserted into the common right femoral artery through a contralateral crossover, showing that the common femoral artery was considered appropriate for a trans-femoral approach due to the good calibre and lack of anterior calcifications. C) 14 French introducer sheath was inserted trans-femorally across the left iliac artery into the aorta without any resistance. D) After the crossing of the aortic valve with 0.035” straight-tip wire with the support of an Amplatz Left catheter, the pre-shaped Super Stiff™ Amplatz wire was exchanged into the left ventricle. E) Pre-dilatation of the aortic valve was performed using an 18×40 mm Z-Med™ balloon (NuMED, Inc., Hopkinton, New York, USA). F) The balloon-expandable aortic valve was unable to cross through the left common iliac artery.
The Heart Team decided to switch to a direct access, with a trans-aortic approach. Trans-aortic access was obtained through upper partial J-shaped sternotomy to the right third intercostal space. Two pledged purse-string sutures were placed at the designated spot, which was identified both by finding a calcific-free spot in the preoperative multidetector CT and by palpating the aorta directly at a minimum distance of 5 cm from the aortic annulus. The insertion of a soft wire and a 6 Fr catheter was performed with a direct needle puncture (Figure 3A). After the crossing of the aortic valve with the wire, a pre-shaped Super Stiff Amplatz guidewire (Boston Scientific, Marlborough, Massachusetts, USA) was placed in the LV, followed by insertion of the 14 Fr expandable insertion sheath into the ascending aorta (Figure 3B). After the deployment, X-ray fluoroscopy demonstrated that the prosthesis had moved partially into the LV outflow tract (Figures 3C, D). The patient fell into cardiopulmonary arrest. After a few minutes of resuscitation with pharmacological agents and internal cardiac massage, her vital signs improved but remained imperfect with wide pulse pressure. The cardiothoracic surgical team performed open midline sternotomy and cardiopulmonary bypass and found a stressed, distended LV that may be attributed to acute aortic regurgitation. Due to heavy calcification of the ST junction, aortic root, the cardiothoracic surgeon could mount 17 mm of the aortic prosthetic valve. The patient was unable to be resuscitated and died due to persistent hypotension and electro-mechanical dissociation, notwithstanding attempts by the heart team.
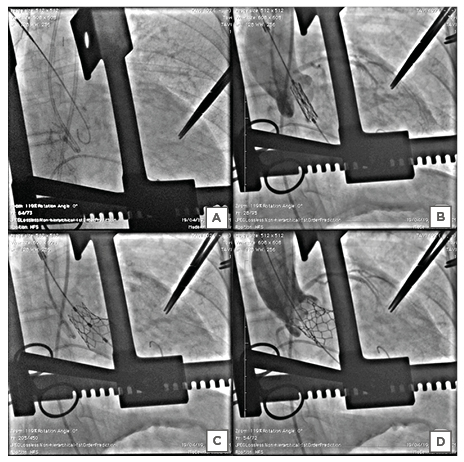
Figure 3: Stepwise procedure of the direct trans-aortic transcatheter aortic valve implantation. A) The Super Stiff™ Amplatz wire was crossed across the aortic valve. B) The optimal positioning of the transcatheter heart valve before the deployment. C) Upon dilatation of the lead to uneven expansion of the balloon-expandable transcatheter heart valve, the proximal end of the valve remained unexpanded while the distal end of the valve expanded fully. D) Aortic root angiography revealed migration of the transcatheter heart valve to the left ventricular outflow tract with electromechanical dissociation, leading to cardiovascular collapse.
DISCUSSION
A valve that migrates to the ventricle unnecessarily can be associated with adverse effects such as mitral insufficiency, arrhythmia, or aortic injury. Prosthesis embolisation shortly after deployment is generally the result of a grave error in the placement or ejection of the system during deployment via an appropriate ventricular contraction.3
Malpositioning of the valve is often recognised as an important factor leading to the occurrence of paravalvular aortic regurgitation as well as weak haemodynamics of the THV, compromise of the mitral valve, and abnormalities in conduction.4 Precise balloon-expandable valve placement requires knowledge of the motion and shortening of the THV during implantation. Acute severe aortic insufficiency can cause hypotension and shock following TAVR. Hypotension and wide pulse pressure on the arterial trace may suggest the diagnosis, with failure to maintain good diastolic pressure after TAVR. Major transvalvular regurgitation after TAVR is rare and is typically associated with acute failure of the structural valve. This may include rupture of the prosthesis or malfunctioning leaflet (‘frozen leaflet’), which is rare but a possible complication following TAVR. Alternatively, to solve the problem, prompt cardiopulmonary bypass and surgical valve replacement may be necessary.3 After its introduction, the trans-aortic procedure has been well adopted by heart teams and has become a viable choice for severe vascular disease that impedes trans-femoral transcatheter aortic valve implantation (TAVI), with 95% procedural success and 2.1% conversion rate to surgical aortic valve replacement as in the present case.5
Kim et al.6 studied periprocedural TVEM in their retrospective ‘TRAVEL registry’, which collected data from 26 international centres, finding that it occurred in 0.92% of cases. The use of first-generation prostheses and the existence of a bicuspid aortic valve were independent predictors of TVEM. Bail-out steps included manoeuvring of the THV using snares or various devices (41.0%), the valve in the valve or multiple implantations (83.2%), and transition to surgery (19.0%) as happened in the present case.6
The criteria recently established by the Valve Academic Research Consortium (VARC)-2 may further standardise definitions of post-operative complications.7 Causes of TVEM were recorded by each site after an in-depth review of procedural documentation and angiographic images, and were classified as procedural, structural, or anatomical, due to a sizing failure or unspecified. TVEM that occurred acutely during the intervention was listed as the most common intra-procedural type and 20.5% occurred in the LV, as in the present case. Further, thorough research found that the most common individual causes of TVEM were malpositioning (50.2%), interference (20.9%), post-dilation (5.9%), sizing error (5.1%), and rapid-pacing failure (4.8%), or unknown (5.5%).6
Conversion to surgery occurred in 19.0% of cases of TVEM and the rate was higher in ventricular TVEM than in aortic TVEM (41.1% versus 13.4%, respectively; p<0.001); the surgical conversion rate was compulsory 12.8%, as in the present case. The overall majority of patients needing cardiopulmonary assistance was 21.6% and comprised cardiopulmonary bypass 17.2%, but the result was worse for all the attempts involving conversion to surgery.8
In a study by Makkar et al.,9 the mortality rate associated with TVEM was even higher (46.2%); that may have been attributed to the exclusive use of balloon-expandable systems, which, due to a higher probability of ventricular embolisation, involve a surgical procedure more frequently.9
Too-low positioning in the LV causes the leaflets to be exposed or creates a leaflet overhang, which leads to severe central aortic regurgitation. Although a small quantity of native leaflet overhang is not uncommon, particularly at the commissures of the aortic valve where the leaflet is attached to the ST junction, it may cause complications by leaving a significant amount of calcified, rigid leaflet above the THV. Various studies have enlisted few risk factors for implant embolisation/migration, such as improper sizing of the TAVI valve, limited or non-uniformly distributed calcification of the aortic annulus, inadequate location of the implant too high or too low in the annulus, or post-deployment native leaflet overhang exerting a downward force on the prosthesis.10
The cases of TVEM in the LV outflow tract were predominantly related to the use of the balloon-expandable SAPIEN XT prosthesis (Edwards Lifesciences, Irvine, California, USA), similar to the present case. Free embolisation into the LV is almost impossible due to the use of self-expanding THVs. With repositionable prostheses, the risk of embolisation or migration can be reduced further.10,11
Intra-procedural TVEM was associated with increased acute and mid-term mortality and morbidity. Notably, a large proportion of TVEM tended to be preventable. But there are few unpreventable causes secondary to technical issues or complex anatomy as in our case.6
The authors believe that the low implantation coincided with severe asymmetric calcification of the ST junction. Non-uniformly dispersed calcification may prevent adequate anchoring and full expansion of the prosthesis. Furthermore, it should be remembered that the retrograde force on a closed valve during diastole was shown to be approximately 10-fold the antegrade force during systole, which would have contributed to the dislocation towards the LV.12
KEY LEARNING POINTS
TVEM is an unlikely circumstance; prevention requires several considerations:
- Careful pre-procedural co-ordination, including precise assessment of the aortic annulus using multidetector CT as a benchmark.
- Choice of the most suitable prosthesis shape and size for the specific patient.
- Appropriate implantation strategies.
- Ensuring proper temporary pacing.
- Effective closed-loop communication between operators, assistants, and technicians.13,14
The overwhelming majority (94.5%) of patients had a specific cause of TVEM, the majority of which were procedure-related, such as malpositioning, manipulation, post-dilatation, and fast-rate pacing malfunction. The authors found three potential causes for this intraprocedural TVEM in the present case: 1) malpositioning, with low valve positioning due to rapid deployment in unstable trans-aortic positioning; 2) inadequate valve expansion; and 3) asymmetric heavy aortic valve calcification.
PERSPECTIVE
Knowledge in TVEM management ought to be a requirement for TAVI teams. Access to cardiothoracic surgery is needed, as is the availability of percutaneous material for THV extraction. Aside from proper TAVI preparation, education, and implementation techniques, advances in delivery systems can reduce the risk of TVEM even further. Later generations of self-expanding and mechanically expanding THVs should have retrieval choices that remain in effect until the prosthesis is implemented. However, due to the inherent presence of THV interference with the native valve system and the lack of suture-based fastening, TVEM will continue to be a challenge of the TAVI procedure.
CONCLUSION
Intra-procedural retrograde migration of the THV is an unusual complication after TAVR. THV displacement into the outflow tract of the LV after deployment is associated with cardiovascular shock and the need for emergency cardiac surgery, and usually has poor outcomes. The gaps in knowledge concerning TVEM are connected not only with clinical outcomes but also with timing, causes, and strategies for bail-outs. Additionally, further research into the re-sheathable new-generation systems may be helpful to avoid such complications in the future.







